Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: Spondyloarthritis Including PsA – Treatment II: PsA
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A. The phase 3 BE COMPLETE study assessed the efficacy and safety of BKZ vs placebo (PBO) in patients (pts) with active PsA and inadequate response to TNF inhibitor (TNFi).
Methods: BE COMPLETE (NCT03896581) comprised a 16-week (wk) double-blind, PBO-controlled period. Pts were eligible if they had a diagnosis of adult-onset, active PsA meeting the Classification Criteria for PsA with duration ≥6 months, ≥3 tender and swollen joints, ≥1 active psoriatic lesion and/or documented history of psoriasis, and inadequate response or intolerance to 1–2 prior TNFi. Pts were randomized 2:1 to subcutaneous BKZ 160 mg every 4 wks (Q4W) or PBO. Primary endpoint: ≥50% improvement in ACR response criteria (ACR50) at Wk 16. Missing data for binary endpoints were imputed as non-response; missing continuous efficacy variables used multiple imputation.
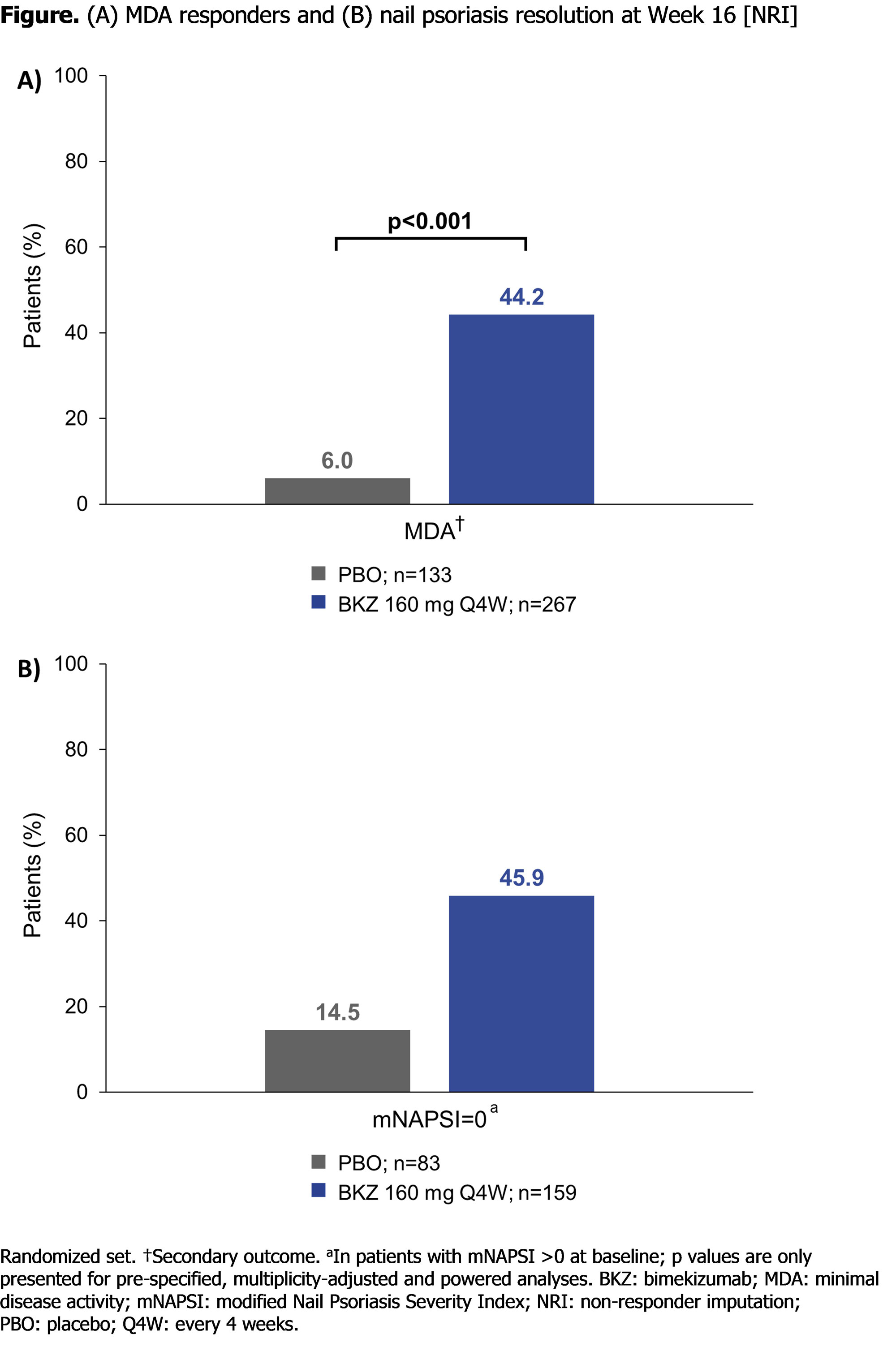
Results: A total of 388/400 (97.0%) pts completed Wk 16 (263/267 [98.5%] BKZ; 125/133 [94.0%] PBO). Baseline (BL) characteristics were comparable between groups. Mean age: 50.5 years; BMI: 29.8 kg/m2; time since diagnosis: 9.5 years; 47.5% male. Mean tender and swollen joint counts were 18.7 and 9.9, respectively; 66.0% had psoriasis affecting ≥3% body surface area (BSA).
At Wk 16, BKZ demonstrated significant improvements vs PBO for the primary endpoint (ACR50: 116 [43.4%] BKZ vs 9 [6.8%] PBO; p< 0.001; Table). Improvement in ACR50 response compared with PBO was observed in the BKZ group as early as Wk 4. Over half of pts with psoriasis affecting ≥3% BSA at BL achieved complete skin clearance at Wk 16 with BKZ (Psoriasis Area and Severity Index [PASI]100: 103/176 [58.5%] BKZ vs 4/88 [4.5%] PBO; Table). BKZ treatment also resulted in greater percentages of pts achieving minimal disease activity (MDA: 118 [44.2%] BKZ vs 8 [6.0%] PBO; p< 0.001) and nail psoriasis resolution compared to PBO (Figure). Further, BKZ demonstrated superiority vs PBO for improvements in physical function, including Health Assessment Questionnaire Disability Index and Short-Form 36-Item Health Survey Physical Component Summary (Table).
To Wk 16, 107/267 (40.1%) pts on BKZ had ≥1 treatment-emergent adverse event (TEAE) vs 44/132 (33.3%) pts on PBO (safety set). Most frequent TEAEs on BKZ were nasopharyngitis (BKZ: 3.7%; PBO: 0.8%), oral candidiasis (2.6%; 0%), and upper respiratory tract infection (2.2%; 1.5%). Two pts on BKZ discontinued due to a TEAE (BKZ: 0.7%; PBO: 0%). Incidence of serious TEAEs was low (BKZ: 1.9%; PBO: 0%); none led to discontinuation. All reported Candida infections were oral; all were mild to moderate, none were systemic. One case of oral candidiasis led to discontinuation. No cases of IBD, major adverse cardiac events, uveitis, serious hypersensitivity, or deaths were reported.
Conclusion: Dual inhibition of IL-17F in addition to IL-17A with BKZ in pts with active PsA and inadequate response to TNFi resulted in clinically meaningful and statistically significant improvements in efficacy outcomes vs PBO. BKZ was well tolerated; no new safety signals were observed.1,2
References: 1. Ritchlin CT. Lancet 2020;395(10222):427–40; 2. Coates LC. Ann Rheum Dis 2021;80:779–80(POS1022).
To cite this abstract in AMA style:
Merola J, Landewé R, McInnes I, Mease P, Ritchlin C, Tanaka Y, Asahina A, Behrens F, Gladman D, Gossec L, Warren R, Ink B, Assudani D, Bajracharya R, Coarse J, Coates L. Bimekizumab Treatment in Patients with Active Psoriatic Arthritis and Inadequate Response to Tumor Necrosis Factor Inhibitors: 16-Week Efficacy and Safety from a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-treatment-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-to-tumor-necrosis-factor-inhibitors-16-week-efficacy-and-safety-from-a-phase-3-randomized-double-blind-pla/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-treatment-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-to-tumor-necrosis-factor-inhibitors-16-week-efficacy-and-safety-from-a-phase-3-randomized-double-blind-pla/