Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: PsA imparts a substantial burden on patient health-related quality of life (HRQoL).1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has shown efficacy and tolerability up to 16 weeks (wks) in patients (pts) with active PsA in the phase 3 BE OPTIMAL and BE COMPLETE studies.2,3
This analysis aimed to assess impact of BKZ treatment vs placebo (PBO) on HRQoL in pts with active PsA that are biologic DMARD (bDMARD)-naïve or had inadequate response to TNF inhibitors (TNFi-IR).
Methods: BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) are phase 3 studies of BKZ in bDMARD-naïve or TNFi-IR pts with PsA, respectively. In BE OPTIMAL, 852 pts were randomized 3:2:1 to subcutaneous (sc) BKZ 160 mg every 4 wks (Q4W), placebo (PBO), or a reference arm (sc adalimumab 40 mg every 2 wks); in BE COMPLETE, 400 pts were randomized 2:1 to sc BKZ 160 mg Q4W or PBO. Primary endpoint in both studies was ACR50 at Wk 16. Measures of function, HRQoL, and utility (pt preference attached to health status)4 included, but were not limited to, Health Assessment Questionnaire Disability Index (HAQ‑DI) (pts with minimal clinically important difference [MCID, ≥0.35 reduction from baseline (BL)], normative state [score ≤0.5],5,6 and change from BL [CfB]), Short-Form 36-Item Health Survey (SF‑36) Physical Component Summary (PCS), PsAQoL, and EuroQol 5-Dimensions 3-Level visual analog scale (EQ-5D-3L VAS). Non‑responder/multiple imputation (NRI, MI) were used for missing binary/continuous variables.
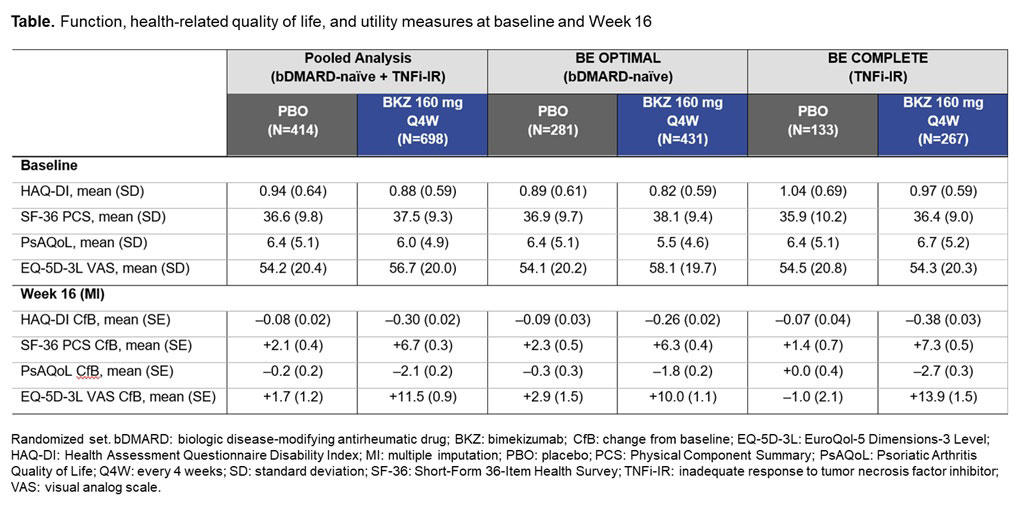
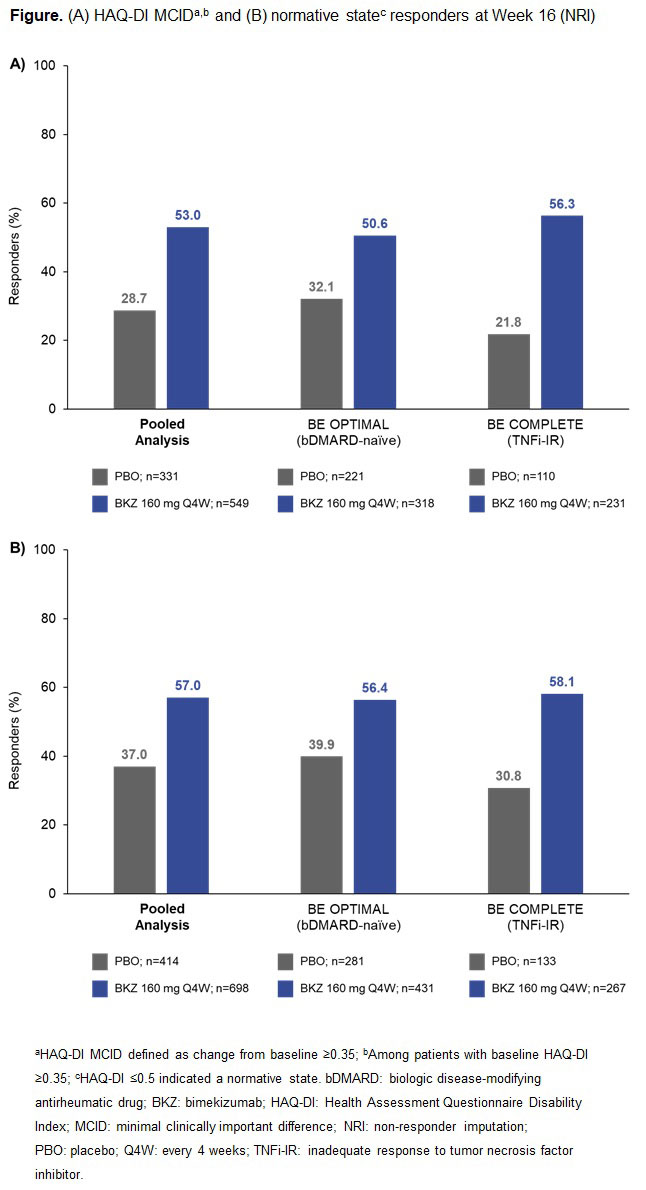
Results: A total of 1,073/1,112 (96.5%) pts randomized to BKZ or PBO completed Wk 16 in BE OPTIMAL and BE COMPLETE. Patient demographics and BL function, HRQoL, and utility scores were generally comparable between PBO and BKZ arms within and across trials (Table).2,3 At Wk 16 (pooled), BKZ-treated pts showed greater improvements in physical function vs PBO‑treated pts, including for key outcomes HAQ-DI MCID (BKZ: 53.0%, PBO: 28.7%) and HAQ-DI ≤0.5 (BKZ: 57.0%, PBO: 37.0%) (Figure), as well as for CfB in norm-based SF‑36 PCS (Table). BKZ-treated pts also showed greater improvements at Wk 16 in HRQoL (PsAQoL CfB; BKZ: –2.1, PBO: –0.2) and utility (EQ-5D-3L VAS CfB; BKZ: +11.5, PBO: +1.7) (Table). BKZ results were similar between bDMARD-naïve and TNFi-IR pts in BE OPTIMAL and BE COMPLETE, respectively (Table).
Conclusion: BKZ treatment resulted in clinically meaningful improvements in measures of HRQoL, physical function, and utility compared with PBO in pts with active PsA. Across measures, results were consistent between bDMARD-naïve and TNFi-IR pts.
References: 1. Gudu T. Expert Rev Clin Immunol 2018;14(5):405–17; 2. McInnes IB. Ann Rheum Dis 2022;81:206–7; 3. Merola JF. Ann Rheum Dis 2022;81:167–8; 4. Bakker CH. Patient Educ Couns 1993;20(2–3):145–52; 5. Krishnan E. Arthritis Rheum 2004;50(3):953–90; 6. Keystone EC. J Rheumatol 2013;40(9):1487–97.
To cite this abstract in AMA style:
Gladman D, Erik L, Thaci D, Gisondi P, Gossec L, Husni M, Gottlieb A, Dobashi H, Ink B, Assudani D, Bajracharya R, Coarse J, Lambert J, Tillett W. Bimekizumab Treatment Improves Health-Related Quality of Life in Biologic DMARD-Naïve and TNFi-IR Patients with Active PsA: Pooled 16-Week Results from Two Phase 3 Randomized, Placebo-Controlled Studies [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-treatment-improves-health-related-quality-of-life-in-biologic-dmard-naive-and-tnfi-ir-patients-with-active-psa-pooled-16-week-results-from-two-phase-3-randomized-placebo-controlled-studi/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-treatment-improves-health-related-quality-of-life-in-biologic-dmard-naive-and-tnfi-ir-patients-with-active-psa-pooled-16-week-results-from-two-phase-3-randomized-placebo-controlled-studi/