Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Bimekizumab (BKZ), a humanized monoclonal IgG1 antibody that selectively neutralizes interleukin (IL)-17A and IL-17F, has shown clinical improvements in joint and skin outcomes over 48 weeks (wks) in patients (pts) with active psoriatic arthritis.1 The primary outcome of ACR50 response at Wk 12 of the dose-ranging BE ACTIVE study was achieved by 24–46% of pts across dosing arms.1 Due to the chronic nature of PsA, maintaining a long-term treatment response at high levels of disease control is particularly important. We assess maintenance of response over approximately 2 years of BKZ treatment in pts who demonstrated a response to BKZ at Wk 12.
Methods: Pts who completed 48 wks of the BE ACTIVE study (NCT02969525; design described elsewhere)1 without meeting withdrawal criteria, were eligible for BE ACTIVE 2 OLE entry (NCT03347110). BKZ was given at 160 mg every 4 wks (Q4W) regardless of previous dose. Analyses include pts randomized to the three highest BKZ doses at BE ACTIVE study baseline (BL): 160 mg, 160 mg with 320 mg loading dose (LD), and 320 mg. Maintenance of response is reported as the percentage (%) of BKZ-treated pts achieving a response at Wk 12, with maintained response over time. Outcomes include ACR20/50/70, minimal disease activity (MDA) and very low disease activity (VLDA), Disease Activity in PSoriatic Arthritis (DAPSA) remission, and body surface area (BSA) 0% (for those pts with BSA ≥3% at BL). Non-responder imputation (NRI) was used for missing data. Results are presented from Wk 12 (end of double-blind treatment) to Wk 108, or Wk 120 for outcomes not recorded at Wk 108. Rates of treatment-emergent adverse events (TEAEs) are reported for the Safety Set (pts who received ≥1 dose BKZ).
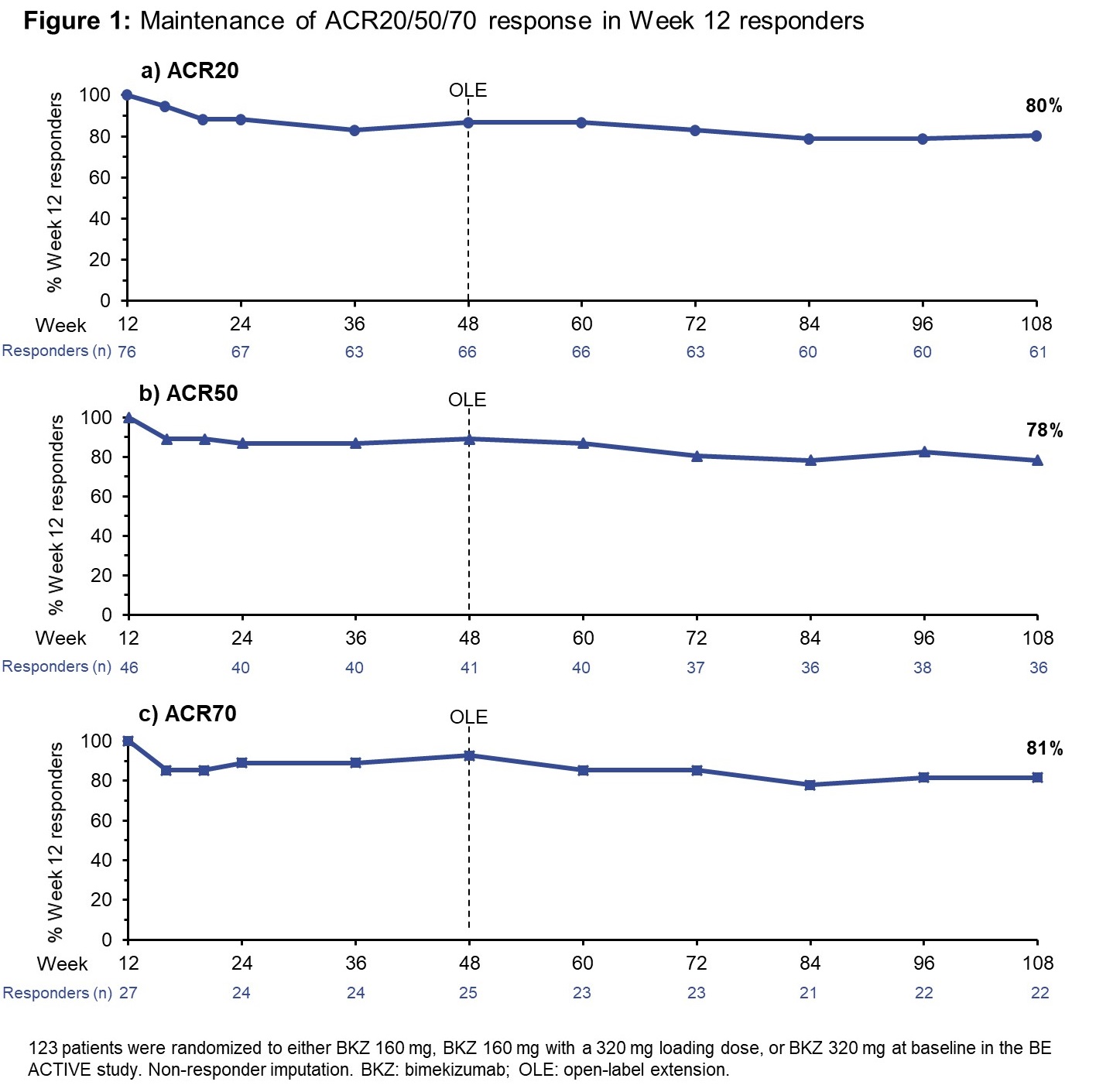
Results: At BL in the BE ACTIVE study, 123 pts were randomized to the three highest BKZ doses (160–320 mg). 109 pts entered the OLE, all receiving BKZ 160 mg Q4W. ACR20/50/70 were achieved by 76 (62%), 46 (37%), and 27 (22%) pts, respectively, at Wk 12 of the double-blind phase. MDA and VLDA criteria were met by 48 (39%) and 18 (15%) pts, respectively; likewise, the number of pts who achieved DAPSA remission and BSA 0% at Wk 12 were 25 (20%) and 32 (40% of the 80 pts with BSA ≥3% at BL), respectively. The majority of pts who achieved responses at Wk 12, across the different outcomes, maintained them to Wk 108 or Wk 120 (Figures 1 and 2). The % of pts achieving ACR20/50/70 at Wk 12 who maintained the response to Wk 108 was 80/78/81%, respectively. MDA and VLDA responses were maintained at Wk 120 by 81% and 72% of Wk 12 responders, respectively; likewise, for DAPSA remission at Wk 108, the response from Wk 12 was maintained by 76%; for BSA 0%: 72%. TEAEs occurred in 87.7% of pts (exposure-adjusted event rate: 181.1/100 patient-years [PY]) and serious TEAEs in 9.3% (4.1/100 PY).
Conclusion: This BKZ-treated population, who achieved high levels of disease control as early as 12 weeks (including ACR50, MDA, VLDA, BSA 0% and DAPSA remission), demonstrated a consistent maintenance of response rate around 80% across both joint and skin outcomes. These results suggest that BKZ provides a robust maintenance of response over at least 2 years of treatment in pts with PsA.
Reference: 1. Ritchlin C. Lancet 2020;395:427–440.
To cite this abstract in AMA style:
Merola J, Behrens F, Kivitz A, Mease P, McInnes I, Ink B, Assudani D, Joshi P, Coarse J, Ritchlin C. Bimekizumab Maintenance of Response in Patients with Psoriatic Arthritis: 2-Year Results from a Phase 2b Dose-Ranging Study and Its Open-Label Extension [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/bimekizumab-maintenance-of-response-in-patients-with-psoriatic-arthritis-2-year-results-from-a-phase-2b-dose-ranging-study-and-its-open-label-extension/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-maintenance-of-response-in-patients-with-psoriatic-arthritis-2-year-results-from-a-phase-2b-dose-ranging-study-and-its-open-label-extension/