Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Psoriatic arthritis (PsA) is a disease with multiple manifestations; it is important that the efficacy of new interventions is assessed by composite endpoints across the spectrum of the disease.1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has shown efficacy, tolerability, and clinical improvement up to 16 weeks (wks) in patients (pts) with active PsA in the phase 3 BE OPTIMAL and BE COMPLETE studies.2,3 To assess the efficacy of BKZ treatment vs placebo (PBO) on disease activity using composite outcome measures in pts with active PsA using pooled data.
Methods: BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) are phase 3 studies assessing BKZ in pts with PsA who are biologic DMARD (bDMARD)-naïve or had inadequate response to TNF inhibitors (TNFi-IR), respectively. Primary endpoint in both studies: proportion of pts with ACR50 response at Wk 16. In BE OPTIMAL (N=852), randomization was 3:2:1 to receive subcutaneous (sc) BKZ 160 mg every four wks (Q4W), PBO, or reference arm (sc adalimumab 40 mg Q2W). In BE COMPLETE (N=400), pts were randomized 2:1 to sc BKZ 160 mg Q4W or PBO. We present pooled and individual study data for BKZ and PBO treatment arms for composite endpoints at Wk 16: minimal and very low disease activity (MDA, VLDA), Disease Activity in Psoriatic Arthritis (DAPSA), Psoriatic Arthritis Disease Activity Score (PASDAS), and ACR50+Psoriasis Area and Severity Index (PASI)100. Non-responder and worst-category imputation (NRI, WCI) were used to account for missing data.
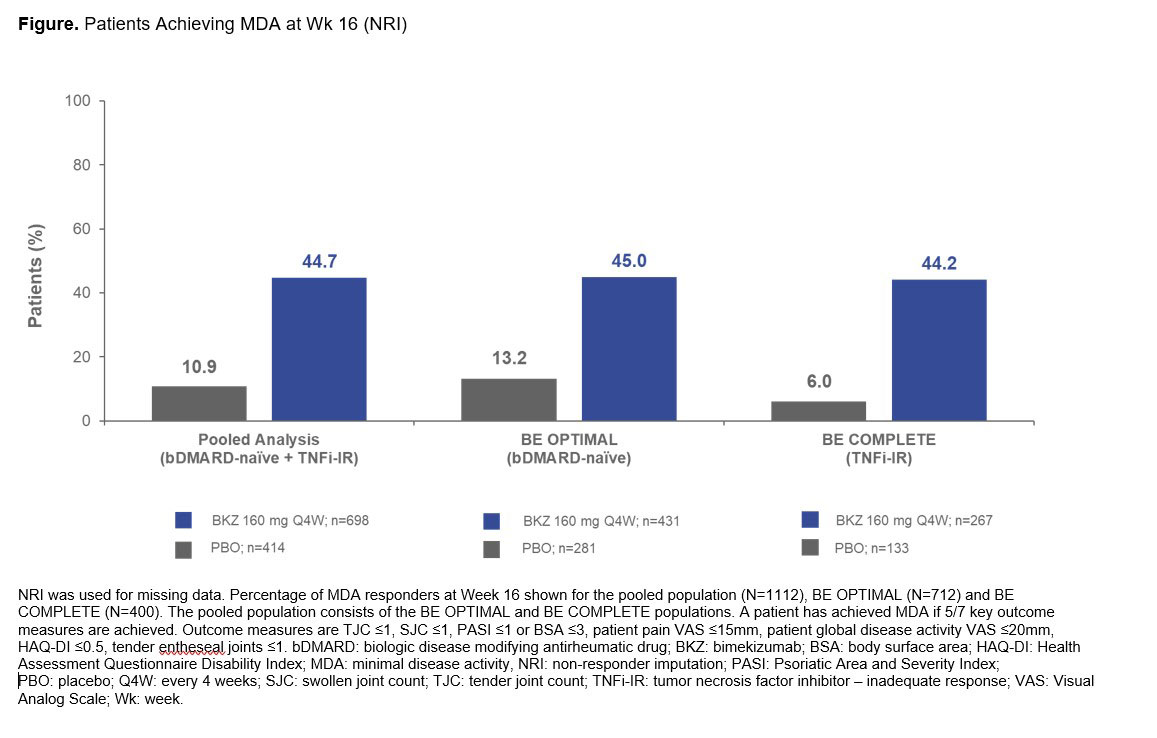
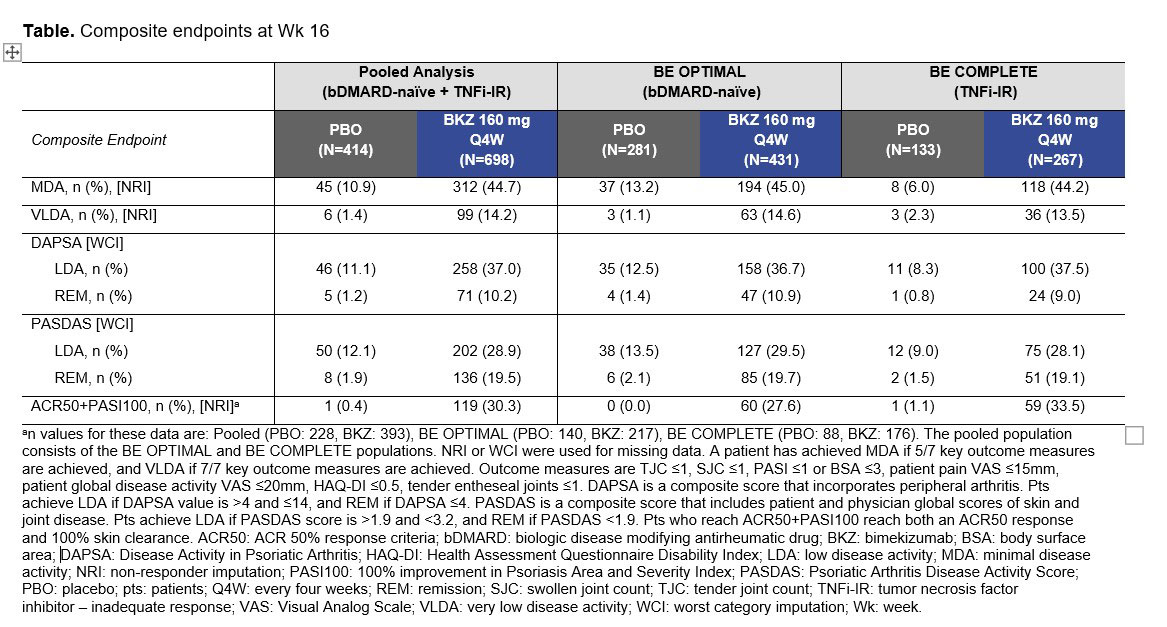
Results: A total of 1,073/1,112 (96.5%) pts randomized to BKZ or PBO completed Wk 16 of BE OPTIMAL and BE COMPLETE. Baseline characteristics were generally comparable between treatment groups and studies. At Wk 16 (pooled), a higher proportion of BKZ-treated pts achieved MDA and VLDA vs PBO‑treated pts (MDA, BKZ: 312 [44.7%], PBO: 45 [10.9%]; Figure; VLDA, BKZ: 99 [14.2%], PBO: 6 [1.4%]). A higher proportion of BKZ-treated pts than PBO achieved low disease activity (LDA) and remission at Wk 16 as measured by DAPSA (LDA, BKZ: 258 [37.0%], PBO: 46 [11.1%]; remission, BKZ: 71 [10.2%], PBO: 5 [1.2%]). Pooled analysis of the composite outcomes PASDAS and ACR50+PASI100 showed that numerically higher proportions of BKZ- vs PBO-treated patients achieved stringent disease activity thresholds; consistent results were observed between each study showing that biologic experienced patients responded as well as biologic naïve patients (Table).
Conclusion: Numerically higher portions of BKZ-treated patients achieved clinically relevant disease activity thresholds compared with PBO in a pooled population at Wk 16 across a series of composite efficacy endpoints covering different domains of PsA. Results were similar between studies, suggesting that BKZ treatment leads to improvements in overall disease activity irrespective of prior bDMARD use.
References. 1. Gladman DD. Rheum Dis Clin North Am 2015;41(4):569–79; 2. McInnes IB. Ann Rheum Dis 2022;81:206–7; 3. Merola JF. Ann Rheum Dis 2022;81:167–8
To cite this abstract in AMA style:
Mease P, Coates L, Landewé R, McInnes I, Ritchlin C, Atsumi T, Behrens F, Gladman D, Gossec L, Nash P, Ink B, Assudani D, Bajracharya R, Coarse J, Prickett A, Gottlieb A. Bimekizumab Improvements in Efficacy on Disease Activity Assessed via Composite Endpoints in Biologic DMARD-naïve and TNFi-IR Patients with Active PsA: Pooled 16-Week Results from Phase 3 Randomized, Placebo-Controlled Studies [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-improvements-in-efficacy-on-disease-activity-assessed-via-composite-endpoints-in-biologic-dmard-naive-and-tnfi-ir-patients-with-active-psa-pooled-16-week-results-from-phase-3-randomized/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-improvements-in-efficacy-on-disease-activity-assessed-via-composite-endpoints-in-biologic-dmard-naive-and-tnfi-ir-patients-with-active-psa-pooled-16-week-results-from-phase-3-randomized/