Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The Group for Research and Assessment of Psoriasis and PsA (GRAPPA) domain‑based treatment recommendations for PsA focus on six key domains: peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, nail psoriasis, and PsA-related conditions: uveitis and IBD.1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)‑17F in addition to IL‑17A, has demonstrated clinical efficacy up to 1 year (yr) in phase 3 clinical trials of patients (pts) with PsA, and in phase 3 clinical trials of pts with psoriasis and axial spondyloarthritis (axSpA).2–7 Here, BKZ long-term efficacy is shown across GRAPPA core domains up to 2 yrs from phase 3 trials in PsA and axSpA.
Methods: Included pts were randomized to receive subcutaneous BKZ 160 mg or placebo (PBO) every 4 weeks (wks; Q4W) in BE OPTIMAL (NCT03895203; biologic DMARD‑naïve pts with PsA), BE COMPLETE (NCT03896581; pts with PsA who were TNF inhibitor-inadequate responders [TNFi‑IR]), BE MOBILE 1 (NCT03928704; non‑radiographic axSpA) and 2 (NCT03928743; radiographic axSpA, i.e., AS). BE OPTIMAL included a reference arm (adalimumab 40 mg Q2W); pts switched to BKZ at Wk 52 (data not shown).2,3,7
From Wk 16, all PBO-randomized pts received BKZ 160 mg Q4W. BE OPTIMAL Wk 52 and BE COMPLETE Wk 16 completers were eligible for BE VITAL (open‑label extension [OLE]; NCT04009499); BE MOBILE 1 and 2 Wk 52 completers could enter BE MOVING (OLE; NCT04436640).
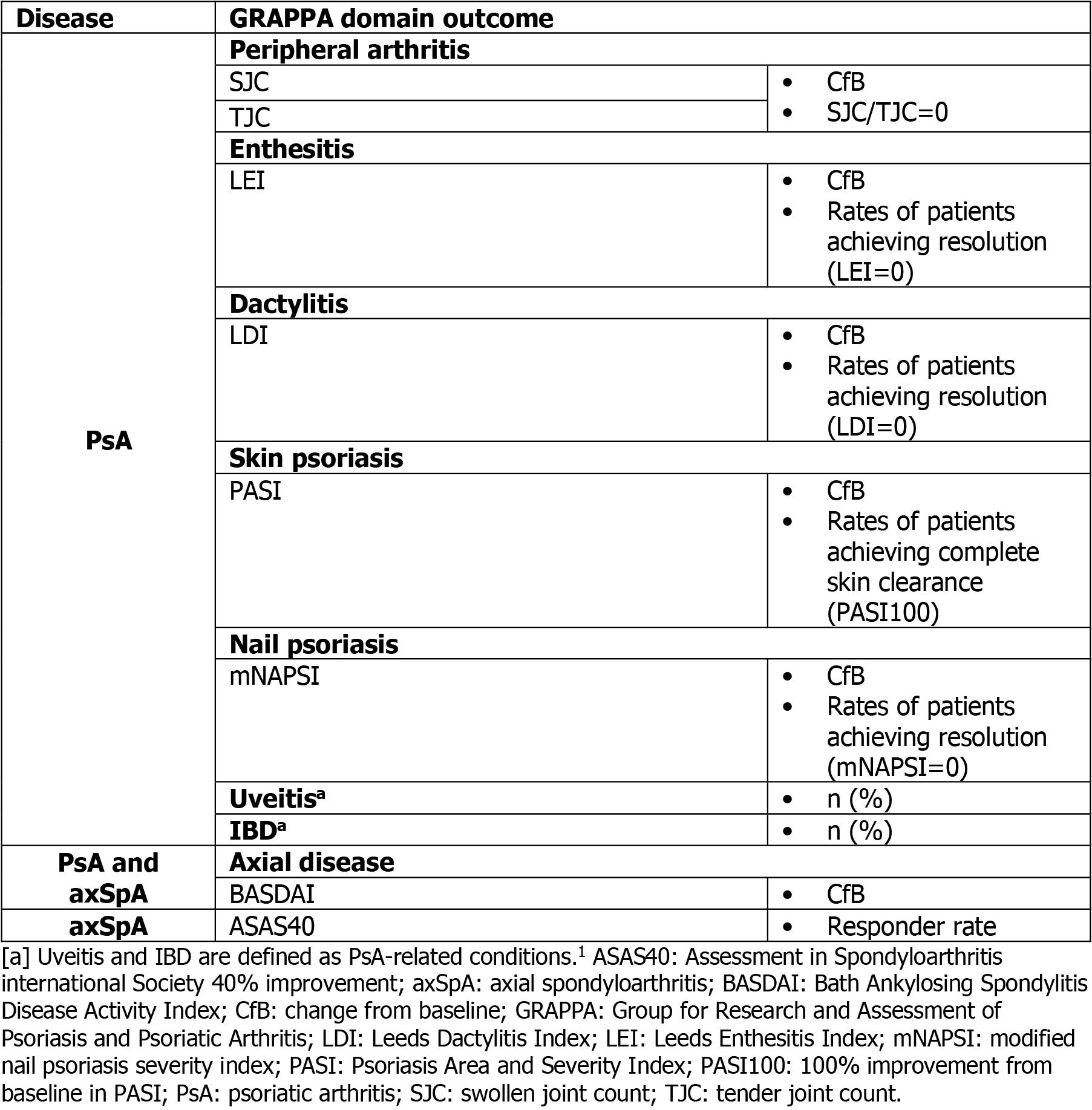
Outcomes are reported by GRAPPA domain (Table 1) to Wk 104 (BE OPTIMAL) and Wk 100 (BE COMPLETE) in PsA; uveitis and IBD reported to Wk 104 (BE OPTIMAL and BE COMPLETE). Axial domain outcomes are reported to Wk 104 (BE MOBILE 1 and 2) in axSpA, in accordance with GRAPPA recommendations.1 Missing data were imputed using non‑responder and multiple imputation (NRI; MI) for binary and continuous outcomes, or reported using observed case (OC).
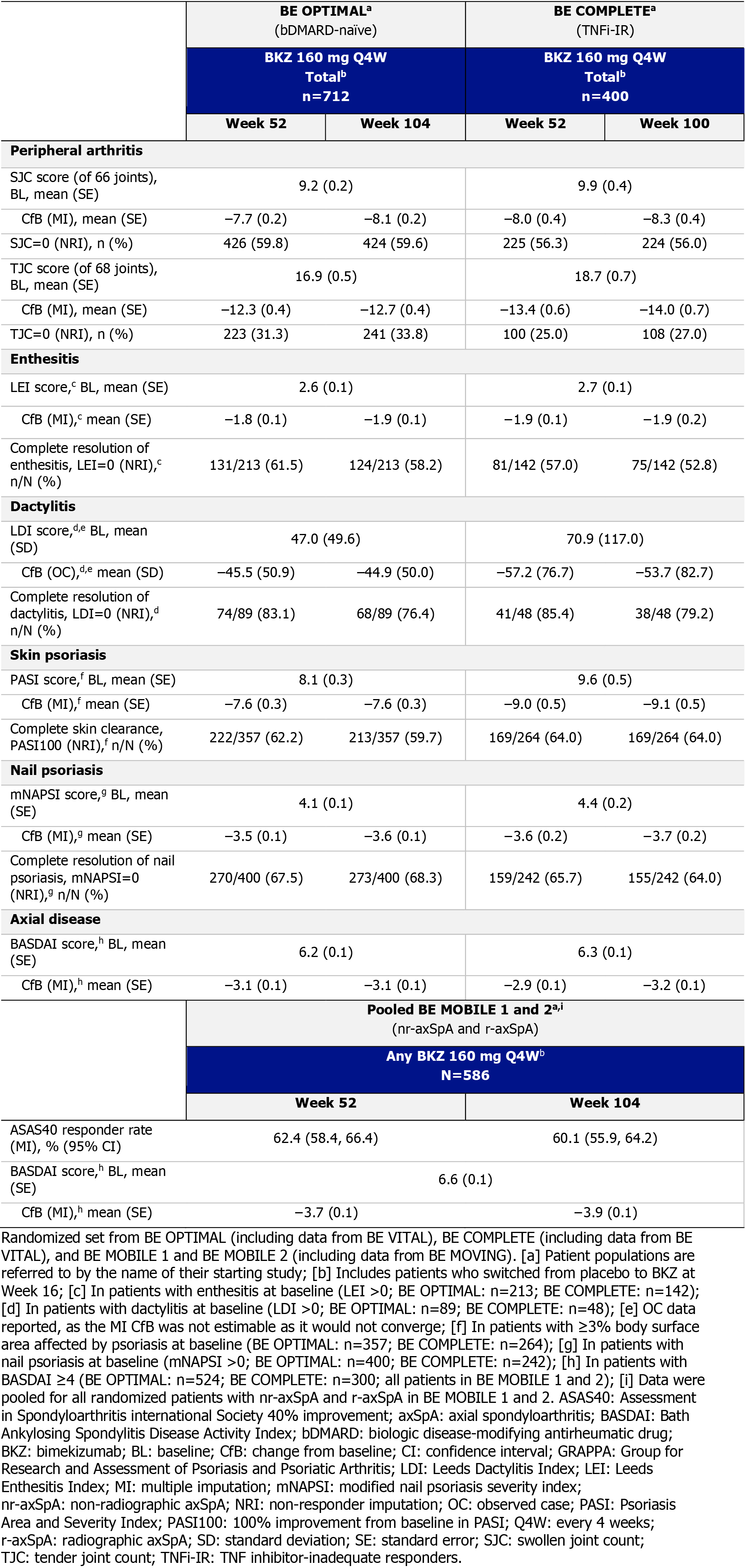
Results: Wk 104/100 completion was similar across all four trials (BE OPTIMAL: 598/712 [84.0%], BE COMPLETE: 322/400 [80.5%], BE MOBILE 1: 189/254 [74.4%], BE MOBILE 2: 267/332 [80.4%]). Baseline demographics and disease characteristics were previously reported.2,3,7
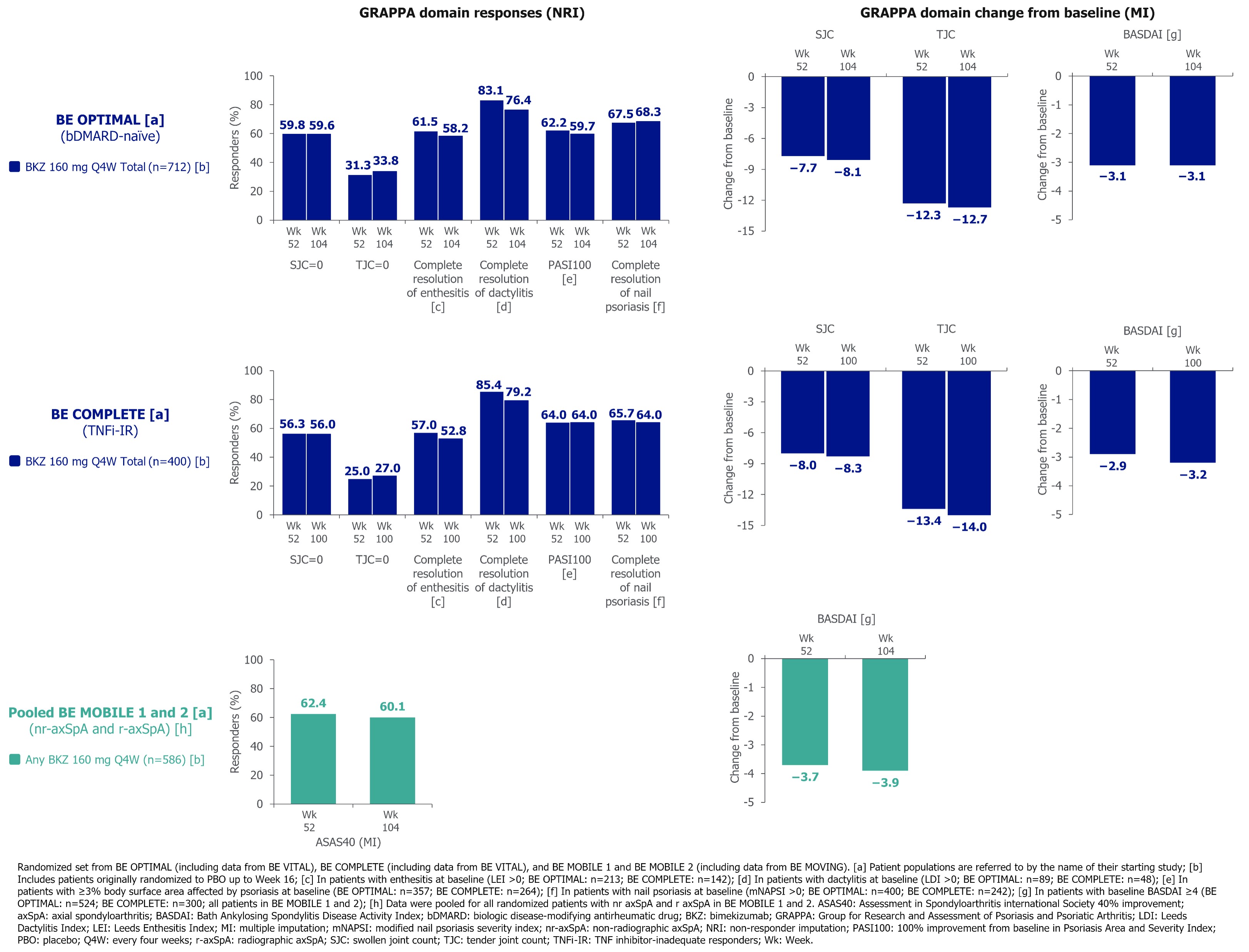
For all GRAPPA domains, 1-yr improvements were sustained to 2 yrs across all studies (Table 2). Individual domain responses were generally consistent between bDMARD‑naïve and TNFi-IR pts (Figure). Improvements in axial domain outcomes were sustained to 2 yrs in BE MOBILE 1 and 2 (Table 2, Figure) and were suggestive of BKZ efficacy for axial disease in PsA.1 To Wk 104, there were no instances of uveitis (BE OPTIMAL; BE COMPLETE). 4 (0.6%) pts in BE OPTIMAL and no pts in BE COMPLETE had definite or probable adjudicated IBD.
Conclusion: Treatment with BKZ resulted in robust improvement maintained up to 2 yrs across GRAPPA domains with low rates of IBD and no uveitis for both bDMARD‑naïve and TNFi‑IR pts with PsA; results from pts with axSpA support efficacy in the axial domain.
References: 1. Coates LC. Nat Rev Rheumatol 2022;18:465–79; 2. Ritchlin CT. Ann Rheum Dis 2023;82:1404–14; 3. Coates LC. RMD Open 2024;10:e003855; 4. Reich K. N Engl J Med 2021;385:142–52; 5. Reich K. Lancet 2021;397:487–98. 6. Warren RB. N Engl J Med 2021;385:130–41; 7. van der Heijde D. Ann Rheum Dis 2023;82:515–26.
To cite this abstract in AMA style:
Merola J, Mease P, Deodhar A, Gottlieb A, Ink B, de Cuyper D, Bajracharya R, Lambert J, Coarse J, Coates L. Bimekizumab Impact on Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Core Domains for Patients with Psoriatic Arthritis: Results up to 2 Years of Treatment Duration [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-impact-on-group-for-research-and-assessment-of-psoriasis-and-psoriatic-arthritis-grappa-core-domains-for-patients-with-psoriatic-arthritis-results-up-to-2-years-of-treatment-duration/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-impact-on-group-for-research-and-assessment-of-psoriasis-and-psoriatic-arthritis-grappa-core-domains-for-patients-with-psoriatic-arthritis-results-up-to-2-years-of-treatment-duration/