Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)‑17F in addition to IL‑17A, has demonstrated clinical efficacy and safety to 3 years (yrs) in patients (pts) with PsA.1,2 Given PsA is a chronic inflammatory condition with a heterogenous clinical presentation,3 it is critical to understand how varying baseline (BL) demographics, disease characteristics, and comorbidities influence treatment efficacy. Here, we assess the impact of BL characteristics on the efficacy of BKZ in pts with PsA at Week (Wk) 16 vs placebo (PBO), and longer-term to 1 yr.
Methods: Pooled post hoc analysis of data from BE OPTIMAL (NCT03895203; biologic DMARD [bDMARD]-naïve) and BE COMPLETE (NCT03896581; TNF inhibitor inadequate response/intolerance [TNFi-IR]). Both studies assessed subcutaneous BKZ 160 mg every 4 wks in pts with PsA and were PBO-controlled to Wk 16, then PBO pts switched to BKZ (PBO/BKZ). BE COMPLETE Wk 16 completers could enter BE VITAL (NCT04009499; open-label extension), in which all pts received BKZ.Efficacy outcomes are reported by BL pt characteristic subgroups. Efficacy outcomes included ≥50% improvement from BL in ACR response criteria (ACR50), the composite outcome minimal disease activity (MDA), complete resolution of swollen joint count (SJC=0), and 100% improvement from BL in Psoriasis Area and Severity Index (PASI100; in pts with BL psoriasis ≥3% body surface area). Data are reported by randomization group at Wk 16; for the BKZ Total group (PBO/BKZ and BKZ-randomized) at Wk 52. The association of BKZ-treated vs PBO pts achieving Wk 16 response overall and for each subgroup was estimated via odds ratios using logistic regression with factors for treatment, study, region, subgroup, and treatment by subgroup interaction (subgroup and interaction excluded in the overall). All p values are nominal and generated for the treatment by subgroup interaction term; missing data imputed using non-responder imputation.
Results: Of the 1,112 pts randomized to PBO (n=414) or BKZ (n=698), 1,073 (96.5%) completed Wk 16 and 1,002 (90.1%) completed Wk 52. Overall BL demographics and disease characteristics were generally similar across the treatment groups (Table). Achievement of ACR50, MDA, SJC=0, and PASI100 responses were greater with BKZ vs PBO within all pt subgroups at Wk 16, with consistent efficacy responses with BKZ treatment for most pt subgroups. Younger pts ( < 45 yrs) treated with BKZ were significantly more likely than older pts (≥45 yrs) to achieve ACR50 (p=0.001), MDA (p=0.006), and SJC=0 (p=0.035) at WK 16. Males were significantly more likely than females to attain ACR50 (p < 0.001). Improved efficacy responses with BKZ treatment were sustained or increased from Wk 16 to Wk 52 across all subgroups (Figure 1–2).
Conclusion: BKZ demonstrated durable and greater improvements in clinical joint, skin, and composite outcomes measures vs PBO at Wk 16, which were sustained to 1 yr, regardless of pt demographics and clinical presentation at BL.References: 1. Gossec L. Ann Rheum Dis 2025:In press; 2. McInnes IB. Ann Rheum Dis 2025:In press; 3. McInnes IB. RMD Open 2024;10:e004494.
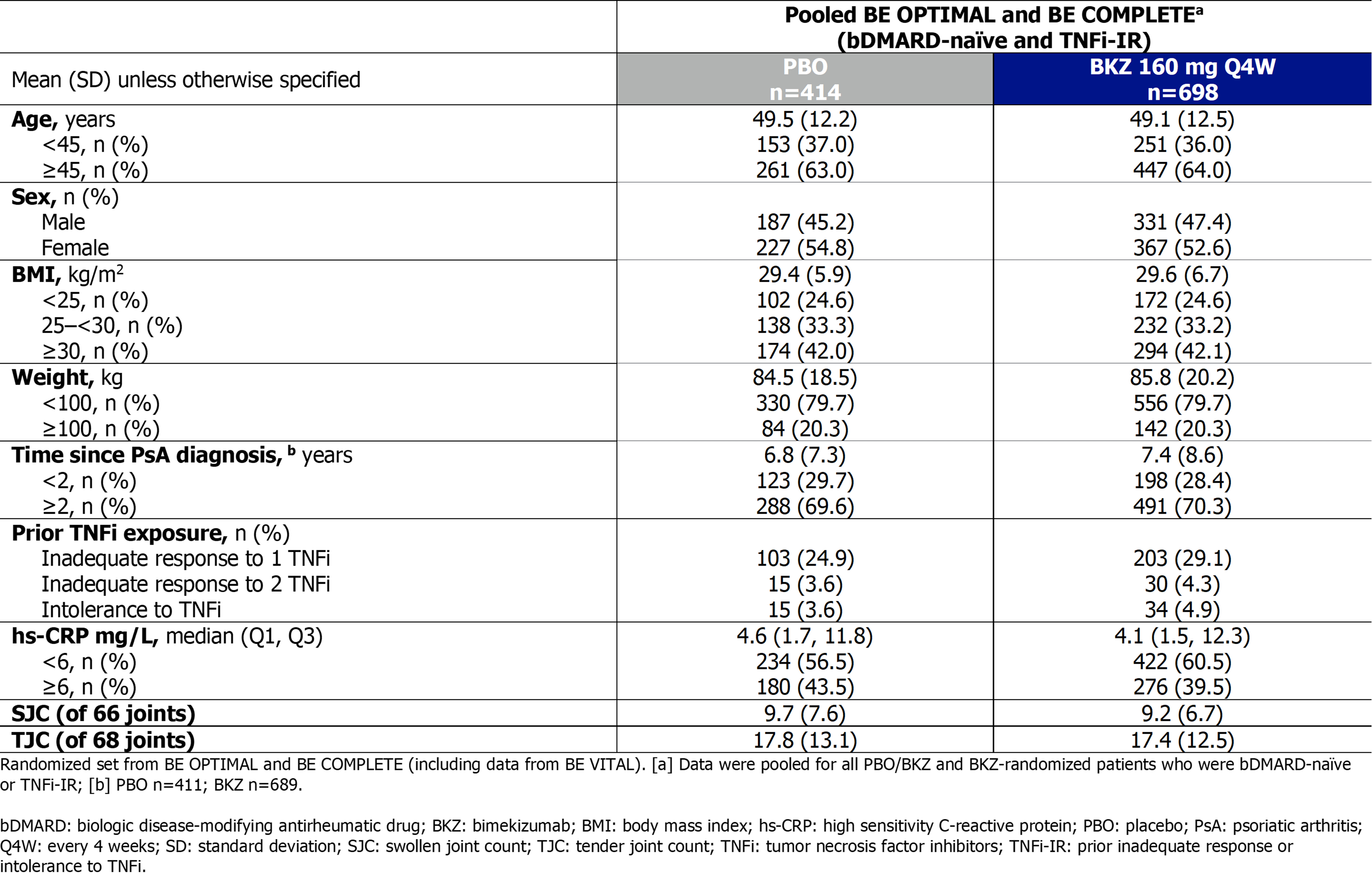 Table. Baseline demographics and disease characteristics in patients
Table. Baseline demographics and disease characteristics in patients
.jpg?2) Figure 1. ACR50 and MDA response rates at Week 16 and Week 52 by baseline demographics and disease characteristics (NRI)
Figure 1. ACR50 and MDA response rates at Week 16 and Week 52 by baseline demographics and disease characteristics (NRI)
.jpg) Figure 2. SJC=0 and PASI100 response rates at Week 16 and Week 52 by baseline demographics and disease characteristics (NRI)
Figure 2. SJC=0 and PASI100 response rates at Week 16 and Week 52 by baseline demographics and disease characteristics (NRI)
Disclosures: L. Eder: AbbVie, 1, 2, 5, 6, BMS, 1, 2, Eli Lilly, 1, 2, 5, 6, Fresenius Kabi, 1, 5, J&J, 1, 2, 5, Janssen, 1, 5, Novartis, 1, 2, 5, 6, Pfizer, 1, 2, 5, UCB, 1, 2, 5; P. Mease: AbbVie, 2, 5, 6, Acelyrin, 2, 5, Amgen, 2, 5, 6, BMS, 2, 5, Century, 2, Cullinan, 2, Eli Lilly and Company, 2, 5, 6, Inmagene, 2, J&J Innovative Medicine, 2, 5, 6, MoonLake Immunotherapeutics, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sana, 5, Spyre, 5, Takeda, 2, UCB, 2, 5, 6; I. McInnes: AbbVie, 2, 6, AstraZeneca, 2, 6, BMS, 2, 5, 6, Boehringer Ingelheim, 2, 5, 6, Cabaletta, 2, 6, Causeway Therapeutics, 2, 6, Celgene, 2, 5, Eli Lilly and Company, 2, 6, Evelo, 2, Gilead, 5, 6, Janssen, 2, 5, 6, MoonLake Immunotherapeutics, 2, 6, Novartis, 2, 5, 6, Pfizer, 5, 6, Sanofi/Regeneron, 5, 6, UCB, 2, 5, 6; M. Husni: AbbVie, 1, 2, Amgen, 1, 2, BMS, 1, 2, Eli Lilly, 1, 2, Janssen, 1, 2, Novartis, 1, 2, Pfizer, 1, 2, UCB, 1, 2; M. Kishimoto: AbbVie, 2, 6, Amgen, 2, 6, Asahi-Kasei Pharma, 2, 6, Ayumi Pharma, 2, 6, BMS, 2, 6, Chugai, 2, 6, Daiichi Sankyo, 6, Eisai, 6, Gilead, 2, 6, Janssen, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Takeda, 2, Tanabe-Mitsubishi, 2, 6, UCB, 2, 6; B. Ink: AbbVie, 11, GSK, 11, UCB, 3, 11; R. Bajracharya: UCB, 3, 11; J. Coarse: UCB, 3, 11; F. Proft: AbbVie, 2, 6, Amgen, 2, 6, BMS, 2, 6, Celgene, 2, 6, Eli Lilly and Company, 2, 5, 6, Galapagos, 2, 6, Hexal, 2, 6, Janssen, 2, 6, Medscape, 2, 6, MoonLake Pharma, 2, 6, MSD, 2, 6, Novartis, 2, 5, 6, Pfizer, 2, 6, Roche, 2, 6, UCB, 2, 5, 6.
To cite this abstract in AMA style:
Eder L, Mease P, McInnes I, Husni M, Kishimoto M, Ink B, Bajracharya R, Coarse J, Proft F. Bimekizumab Demonstrated Early and Sustained Efficacy Regardless of Baseline Characteristics in Patients with Active Psoriatic Arthritis: Pooled Post Hoc Results up to 1-Year from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-demonstrated-early-and-sustained-efficacy-regardless-of-baseline-characteristics-in-patients-with-active-psoriatic-arthritis-pooled-post-hoc-results-up-to-1-year-from-two-phase-3-studies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-demonstrated-early-and-sustained-efficacy-regardless-of-baseline-characteristics-in-patients-with-active-psoriatic-arthritis-pooled-post-hoc-results-up-to-1-year-from-two-phase-3-studies/
