Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Joint involvement, a frequent manifestation of SLE, can be assessed using global lupus disease activity indices (SLEDAI-2K, BILAG-2004) and/or by assessing joint tenderness and swelling (28-joint count).1 This post-hoc analysis evaluated changes in joint manifestations in the LILAC Part A trial of BIIB059 versus placebo (NCT02847598)2 using various definitions.
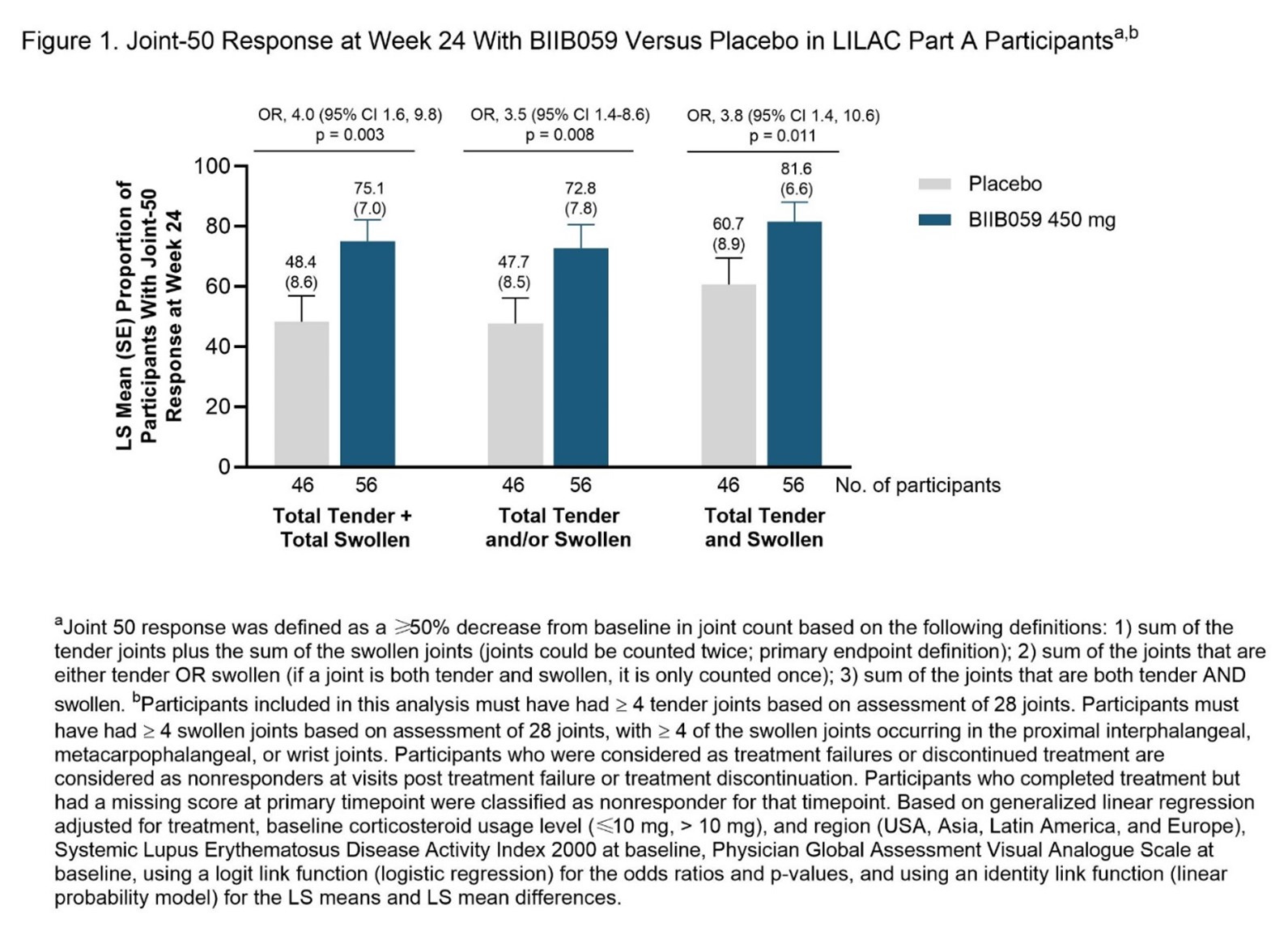
Methods: The randomized, double-blind, placebo-controlled LILAC Part A study enrolled participants who fulfilled 4 of 11 revised ACR 1997 SLE classification criteria3,4 and who had ≥4 tender and ≥4 swollen joints, active skin disease, and positive lupus antibodies (ANA and/or anti-dsDNA). “Joint-50” response was defined as a 50% reduction from baseline in active joint count at Week 24. Three definitions of total active joint count were used: (1) sum of total tender plus total swollen joints; (2) sum of joints that were tender and/or swollen; (3) sum of joints that were both tender and swollen. Arthritis scored on SLEDAI-2K and BILAG-2004 was also analyzed.
Results: The analysis included 56 and 46 participants treated with BIIB059 450mg and placebo, respectively. At Week 24, a greater proportion of BIIB059-treated participants achieved a Joint-50 response compared to placebo; findings were consistent across the 3 different definitions (Figure 1). The percentage of participants with resolution of arthritis by SLEDAI-2K was greater in BIIB059-treated participants (48.2%) versus placebo (21.7%); similar findings were seen with the BILAG-2004 arthritis (musculoskeletal domain) evaluation. Incidence and severity of adverse events were similar with BIIB059 versus placebo in LILAC Part A.
Conclusion: BIIB059 treatment was associated with improvement in SLE arthritis. More participants achieved a Joint-50 response with BIIB059 versus placebo, regardless of how active joint count was defined.
1. Mahmoud. Curr Opin Rheumatol. 2017;29(5):486-492. 2. Furie. Arthritis Rheum. 2020;72(S10):0935. 3. Hochberg. Arthritis Rheum.1997;40(9):1725. 4.Tan. Arthritis Rheum. 1982;25(11):1271-7.
This study was sponsored by Biogen. Funding for medical writing support was provided by Biogen.
To cite this abstract in AMA style:
van Vollenhoven R, Furie R, Kalunian K, Dall'Era M, Werth V, Huang X, Carroll H, Musselli C, Barbey C, Franchimont N. BIIB059 Demonstrates Improvement in Joint Manifestations in Participants with Systemic Lupus Erythematosus in Part a of a Phase 2, Randomized, Double-Blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/biib059-demonstrates-improvement-in-joint-manifestations-in-participants-with-systemic-lupus-erythematosus-in-part-a-of-a-phase-2-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biib059-demonstrates-improvement-in-joint-manifestations-in-participants-with-systemic-lupus-erythematosus-in-part-a-of-a-phase-2-randomized-double-blind-placebo-controlled-study/