Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: To achieve clinical remission/low disease activity in patients with rheumatoid arthritis (RA), the treat-to-target (T2T) guidelines recommend frequent disease monitoring through patient-rheumatologist interactions. The economic implications of treating to target have not been fully quantified. This study aimed to estimate the effects of compliance with T2T recommendations regarding patient follow-up on health care resource utilization (HRU) and medical service costs in RA patients newly initiated on a disease-modifying anti-rheumatic drug (DMARD).
Methods: Patients ≥18 years old with ≥2 RA diagnoses and ≥1 prescription fill for a DMARD that was preceded by a rheumatologist encounter (within 7 days) were identified from the MarketScan® Commercial Claims and Encounters database (1/2000 to 9/2011). A patient’s index date was randomly selected among all new DMARD initiation dates with ≥3 months of continuous enrollment before and ≥15 months after that date. Patients with a follow-up rheumatologist visit within 90 days of the index date were defined as compliant, while all others were classified as noncompliant. HRU and costs (measured from a payer’s perspective in 2010 US dollars) were measured over a 1-year period starting 90 days after the index date. Adjusted all-cause HRU and medical service costs were compared between compliant and noncompliant patients using multivariable regression.
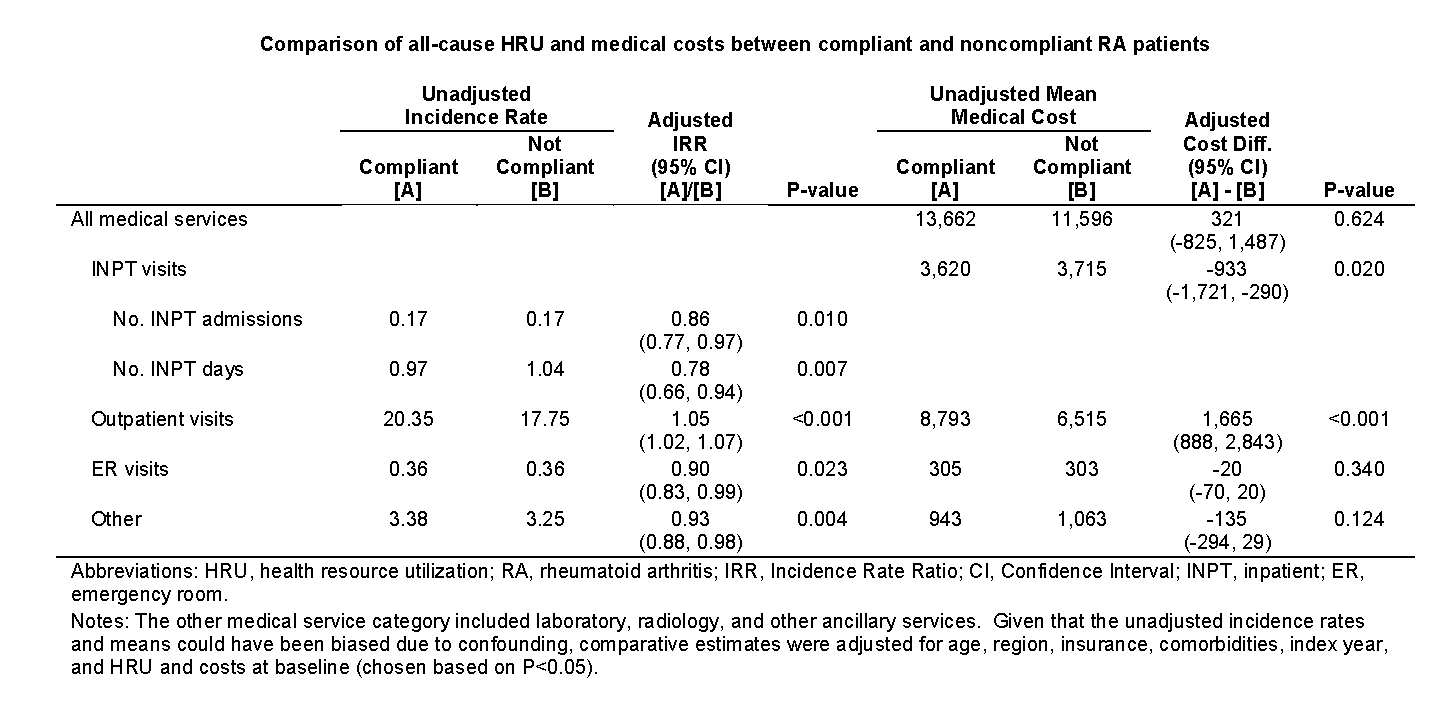
Results: A total of 15,103 RA patients were selected with 10,739 (71.1%) being designated as compliant. The average age was 49.9 years, and 23.2% were male. After adjusting for potential confounding, compliant patients exhibited significantly fewer inpatient admissions and inpatient days, fewer emergency room (ER) visits, and less use of other medical services but significantly more outpatient visits than noncompliant patients. Consequently, compliant patients had significantly lower adjusted inpatient costs but significantly higher adjusted outpatient costs than noncompliant patients. The inpatient cost reduction offset the increase in outpatient costs such that there was no significant difference between the two cohorts with respect to aggregate adjusted medical service costs.
Conclusion: RA patients benefit from being followed in a manner consistent with T2T guidelines. Notably, they exhibit fewer inpatient visits with lower length of stay and lower ER utilization without incurring an increase in medical service costs due to more frequent follow-up with a rheumatologist. However, more research is needed to assess the impact of rigorous follow-up on patient quality of life.
Disclosure:
M. J. Bergman,
None;
J. W. Shaw,
Abbott Laboratories,
3;
M. A. Cifaldi,
Abbott Laboratories,
3,
Abbott Laboratories,
1;
A. Guerin,
Abbott Laboratories,
5;
P. Chopra,
Abbott Laboratories,
5;
J. Signorovitch,
Abbott Laboratories,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/benefits-of-treat-to-target-guideline-compliance-in-patients-with-rheumatoid-arthritis-a-retrospective-claims-analysis/