Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Based on international task force
recommendations, the primary goal of rheumatoid arthritis (RA) treatment is to:
achieve control of symptoms to greatest extent possible, prevent progression of
structural damage, and normalize functional status and social participation (1).
A Current definition of comprehensive disease control (CDC) defined as DAS28
(CRP) <2.6, HAQ DI <0.5, D mTSS (modified Sharp score) ≤0.5 has
been assessed using data from three clinical trials (DE019, PREMIER and OPTIMA)
(2). The objective of this study was to examine the
potential incremental benefit of achieving CDC using real world data from
Corrona, a large observational RA registry in the USA. Because there were not
enough patients who achieved remission but not CDC, data were assessed for
patients who achieved CDC compared to those achieving LDA but not CDC, using
various measures of LDA.
Methods: RA patients ≥18 years of age in
moderate/high disease activity (CDAI>10) with available CDAI, mHAQ and data
on new radiographic progression at index visit and at 6m were included,
regardless of disease modifying anti-rheumatic drug (DMARD) therapy status. Corrona
patients were classified as achieving CDC vs not achieving CDC based on a
previously constructed model including age, gender, disease activity index
(CDAI), modified health assessment questionnaire (mHAQ) and presence of any new
radiographic progression. Patient characteristics at index visit and 6m were
compared between predicted CDC achievers and non-achievers but in LDA as
defined by other measures like CDAI, DAS28-CRP, mDAS and RAPID3.
Results: There were 1627 patients who met the
inclusion criteria of which 20% were CDC achievers (n=331) and 80% non-achievers
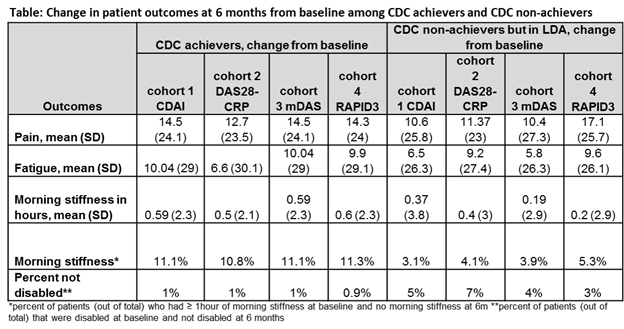
(n=1296). CDC achievers had significantly lower patient reported pain, fatigue,
presence of morning stiffness at baseline, 6m and greater change from baseline.
In addition, fewer CDC achievers reported quality of life problems at 6m from
baseline compared to cohorts who achieved LDA (defined by CDAI, DAS28-CRP, and
mDAS) (Table). However same degree of differences in work status,
patient pain and fatigue and quality of life measures were not seen in the
cohort achieving LDA defined by RAPID3. Patients who achieved LDA (RAPID3)
reported lower patient reported pain and fewer patients were disabled at 6m when
compared to the CDC achievers. (Table).
Conclusion: In this real world analyses, patients who
achieved CDC had significant improvement in clinical, structural and functional
efficacy outcomes at 6 months compared to the patients who did not achieve CDC
but were in LDA defined by CDAI, DAS28-CRP, or mDAS with the exception of
RAPID3, where we observed better outcomes for certain patient reported
measures. Further studies to assess incremental value of CDC over remission
will be valuable.
References: 1. Smolen JS et al. 2010 doi:
10.1136/ard.2009.123919. 2. Emery P et al. 2014 Aug 19. pii:
annrheumdis-2014-205302.
To cite this abstract in AMA style:
Kavanaugh A, Etzel CJ, Malley W, Karki C, Greenberg JD, Bao Y, Chen N, Garg V. Benefits of Achieving Comprehensive Disease Control (CDC) in Patients with Rheumatoid Arthritis: Evidence from the Corrona Registry [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/benefits-of-achieving-comprehensive-disease-control-cdc-in-patients-with-rheumatoid-arthritis-evidence-from-the-corrona-registry/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/benefits-of-achieving-comprehensive-disease-control-cdc-in-patients-with-rheumatoid-arthritis-evidence-from-the-corrona-registry/