Session Information
Session Type: Abstract Submissions
Session Time: 5:30PM-7:00PM
Background/Purpose:
Familial, or primary, hemophagocytic lymphohistiocytosis (pHLH), is a rare but highly fatal condition due to mutations in lymphocyte cytolytic pathway genes. Secondary HLH (sHLH), termed macrophage activation syndrome (MAS) when associated with rheumatic disorders, affects children and adults with various disorders. sHLH can be associated with heterozygous defects in pHLH genes and usually the distinction between both forms is blurred. Both forms are routinely treated with etoposide-based protocols which are frequently limited by significant toxicity and mortality. Alternative less toxic therapies are currently being explored for sHLH. Anakinra is a recombinant interleukin-1 receptor antagonist that has been reported to improve survival in several cases of MAS.
We performed a retrospective chart review of all anakinra-treated sHLH patients at Children’s of Alabama from January 2008 to December 2016. The treating physician assigned diagnosis of sHLH was evaluated using 5 different sets of criteria: HLH-2004 and -2009, lupus (SLE) MAS, systemic juvenile idiopathic arthritis (sJIA) MAS-2016, and the H-Score. Demographic, clinical, laboratory, concurrent treatment, and outcome data were collected and analyzed by appropriate univariate statistical approaches.
Results:
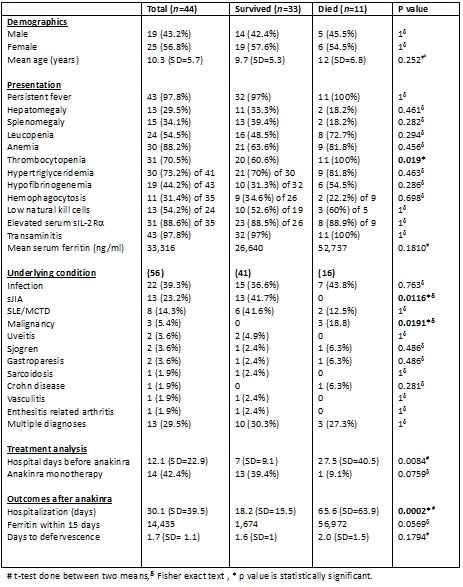
44 (25 female) sHLH patients (mean age 10.3 years, range 1 – 19) were treated with anakinra. The underlying and associated diseases were sJIA (13), SLE/MCTD (8), infection (22), m
alignancy (3), gastroparesis (2), and other (6). Median duration of hospitalization was 15.5 days. The overall mortality was 25%. Earlier start of anakinra was associated with improved survival (p =0.008).
Mean pre-treatment ferritin level was 33,316 ng/ml and dropped to 14,435 (43% decrease) within 15 days of starting anakinra. Mean duration to defervescence after anakinra was 1.7 days. Thrombocytopenia <100,000/µL was associated with increased mortality (p=0.019). sJIA associated MAS had 100% survival, whereas sHLH with underlying malignancy had 100% mortality.
Conclusion:
Anakinra appears to be effective for treating non-malignancy sHLH in children, particularly those with sJIA. The study is limited by its retrospective nature, non-uniform approach to therapy, lack of treatment controls, and variable follow-up period. Anakinra is currently being studied in a randomized, double-blinded, placebo-controlled trial to treat sHLH in children and adults.
To cite this abstract in AMA style:
Eloseily EMA, Weiser P, Haines H, Mannion M, Stoll ML, Beukelman T, Atkinson P, Cron RQ. Benefit of Anakinra in Treating Pediatric Secondary Hemophagocytic Lymphohistiocytosis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 4). https://acrabstracts.org/abstract/benefit-of-anakinra-in-treating-pediatric-secondary-hemophagocytic-lymphohistiocytosis/. Accessed .« Back to 2017 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/benefit-of-anakinra-in-treating-pediatric-secondary-hemophagocytic-lymphohistiocytosis/