Session Information
Date: Sunday, November 8, 2015
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment Poster Session I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: To investigate the efficacy and safety of belimumab in patients
affected with active systemic lupus erythematosus (SLE) refractory to standard
therapy.
Methods: Fifty-eight
patients, 54 female and 4 male, mean age 39.9±10.7 years, mean disease duration
13.1±8.3 years, affected with SLE (ACR criteria), with active disease
manifestations and active serology (low C3/C4 and high dsDNA) unresponsive to
standard therapy were treated with belimumab (10 mg/kg at day 0, 14 and 28 and
then every 28 days). SLEDAI-2K, SLICC/ACR damage index (SDI), anti-dsDNA, C3
and C4 serum levels, and prednisone daily dose were recorded at baseline, at
month 3, 6, 9, 12, and every 6 months thereafter. SDI calculated 5 years before
the initiation of belimumab was also considered. DAS-28, 24-hour proteinuria,
CLASI (Cutaneus LE Disease Area and Severity Index), blood cell count were
recorded to evaluate organ response to belimumab. SLE flares were defined by
SLEDAI flare index and flare rate was reported as number of flare/100 patients/year.
SLE flare rate was evaluated in the
12 months before and after belimumab initiation.
Adverse events (AEs) were recorded at every
clinical evaluation and were defined as follow: infectious
AEs, non-infectious AEs, infusion or hypersensitivity reactions. AE were
considered severe (SAEs) in case of hospitalization
and/or death and/or life-threatening manifestations.
Results: Mean follow-up period was 14.1±7.7 months. Refractory
manifestations requiring the use of belimumab as add on therapy were
musculoskeletal (35.9%), mucocutaneous (27.2%), renal (22.1%), hematologic (11.7%),
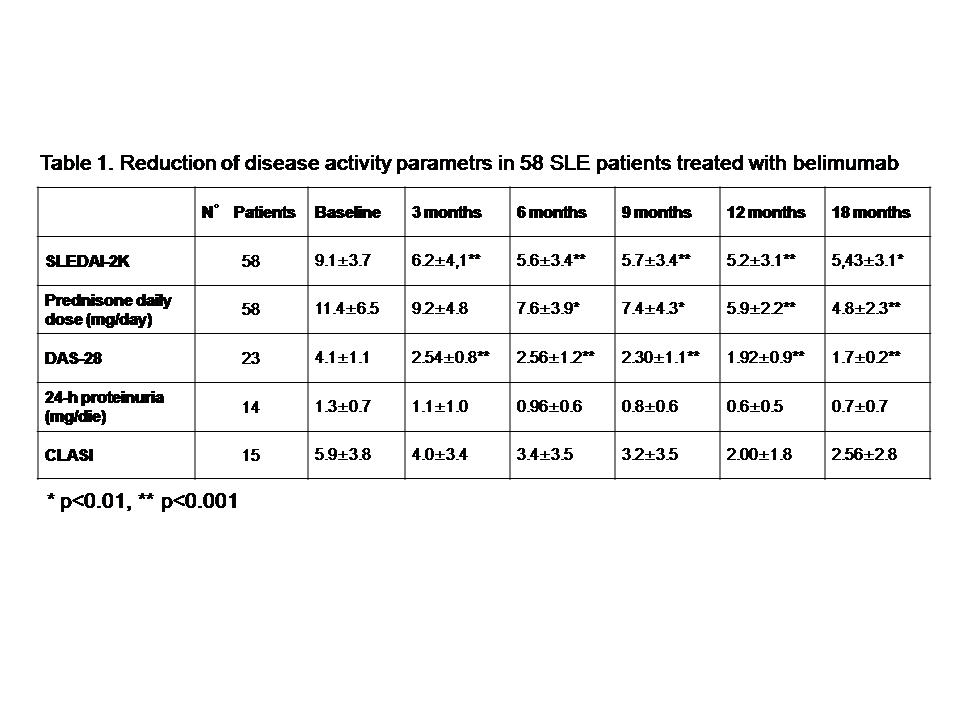
and constitutional (3.1%). At 3, 6, 9, 12, and 18 months of follow-up SLEDAI-2K,
prednisone daily dose and DAS-28 significantly declined compared with baseline
(Table 1). Moreover, a reduction of 24-hour proteinuria and CLASI (Table 1) and
an increase in C3 and C4 values, although not significant, was also found. We
observed 82 flares/100 patients/year before and 37 flares/100 patients/year (p=0.0001)
after belimumab initiation. SDI was 0.62±0.65 five years before belimumab
initiation, 0.86±1.03 at baseline and 0.86±1.03 after 18 months of follow-up.
One hundred fifty-one AEs were observed.
Thirty five patients (60.3%) experienced ≥1AEs. Infectious AEs, non-infectious AEs, infusion and
hypersensitivity reactions were 50.4%, 17.2%, 1.3%
and 31.1% of total AEs,
respectively. Two SAEs were observed, one non-infectious
(deep vein thrombosis) and one infectious AEs
(pneumonia). No severe infusion reactions and no discontinuation
due to AEs were observed.
Conclusion: Belimumab was effective and safe in a clinical
practice setting. Notably, belimumab reduced the number of flares and prevented
the progression of damage in our patients with active SLE.
To cite this abstract in AMA style:
Iaccarino L, Bettio S, Reggia R, Zen M, Frassi M, Andreoli L, Nalotto L, Gatto M, Pea L, Bassi N, Larosa M, Zanola A, Punzi L, Tincani A, Doria A. Belimumab Reduces the Frequency of Flares and Prevents Damage Progression in SLE Patients: Experience in a Clinical Practice Setting [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/belimumab-reduces-the-frequency-of-flares-and-prevents-damage-progression-in-sle-patients-experience-in-a-clinical-practice-setting/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/belimumab-reduces-the-frequency-of-flares-and-prevents-damage-progression-in-sle-patients-experience-in-a-clinical-practice-setting/