Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Lupus nephritis (LN) ultimately requires renal replacement therapy (RRT) in 10-30% of LN patients. Thirty percent of these patients receive a kidney transplant. Belatacept is a second-generation, selective, T-cell co-stimulator blocker (inhibits CTLA-4) used as an alternative to calcineurin inhibitors (CNI) for maintenance regimens after kidney transplants. The pathogenic relevance of CTLA-4 inhibition and the favorable cardiovascular profile of belatacept make it an attractive therapeutic option in SLE. Additionally, intravenous administration of belatacept ensures therapeutic adherence.
Methods: This retrospective, single-center study evaluates the outcomes of LN kidney transplant recipients treated with belatacept from 2006 – 2018 at the Columbia University Lupus and Renal Transplant Cohorts. The treatment regimen consisted of 5mg/kg belatacept every 2 weeks for a total of 10 weeks (5 doses total), followed by monthly dosing. CNI weaning among the belatacept group was not standardized. Immunosuppressive regimen, kidney allograft function, and SLE activity were examined.The current study was initiated to evaluate the effect of belatacept on graft function and extrarenal SLE.
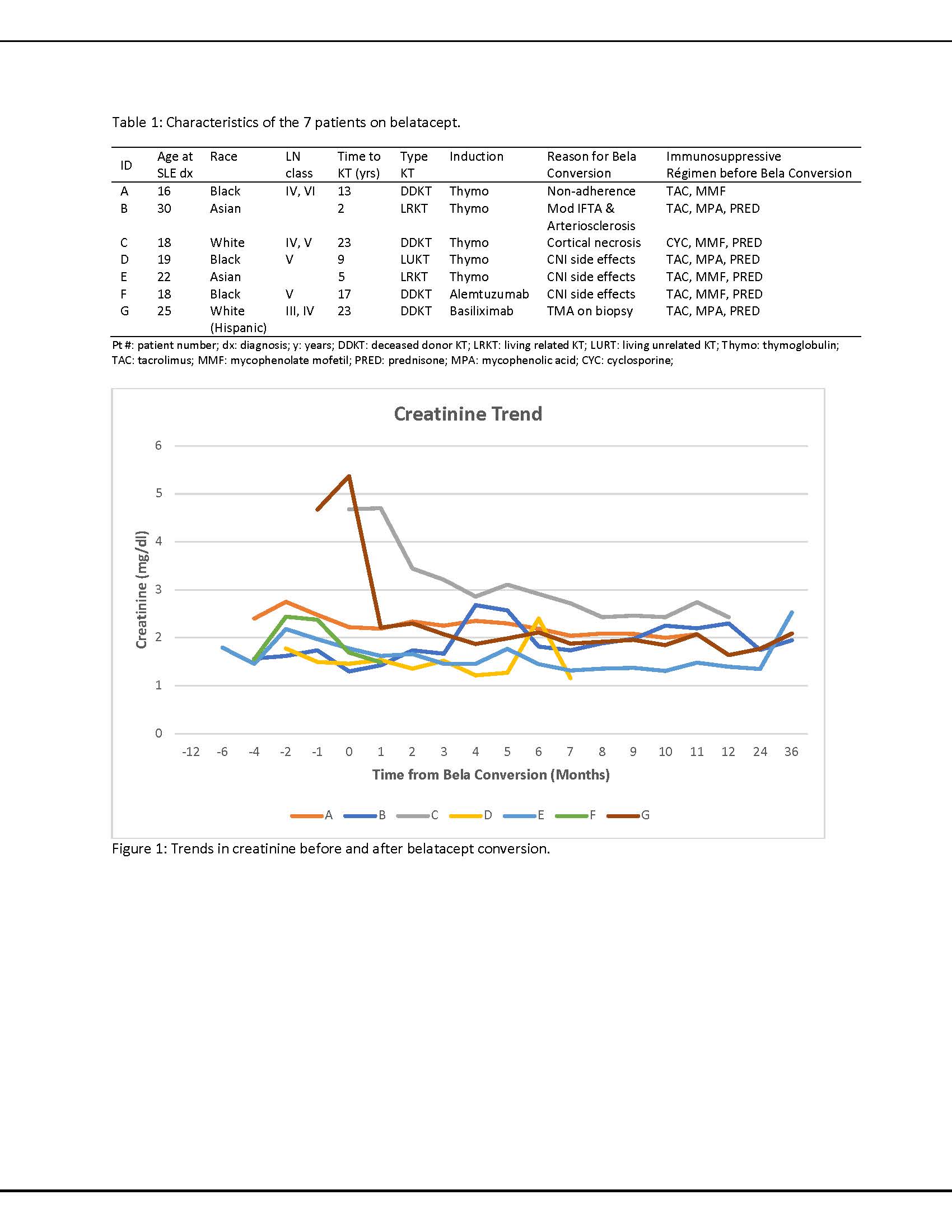
Results: Forty-eight patients with LN had undergone a kidney transplant between 2006 and 2018 with a mean follow-uptime of 72.2 ± 74.6 months. Belatacept was started in seven patients on CNI regimens (TAC n=6, cyclosporine n=1) at 15.5 ± 17.1 months following kidney transplantation. All patients were female with a mean age at SLE diagnosis of 21.1 ± 4.9 years. Five patients had undergone RRT prior to kidney transplantation (4 hemodialysis, 1 peritoneal dialysis) for 38.7 ± 37.8 months. The mean interval between SLE diagnosis and KT was 13.1 ± 8.3 years. At the time of belatacept initiation, all patients were also treated with prednisone (7.1 ± 2.7 mg/day). Six were additionally treated with mycophenolate (1123 ± 625 mg/day), and one was additionally treated with azathioprine (25mg/day). CNIs were continued in five of seven patients at six months after belatacept. Two patients were on hydroxychloroquine, 2 took it only prior to KT. In five patients, creatinine levels stabilized six months after belatacept, one returned to hemodialysis due to CNI-toxicity and pyelonephritis and one is relisted for a kidney transplant due to ACR and cortical necrosis(Fig. 1). No allograft failure due to recurrent LN was noted in any of the patients. Five patients are currently followed for extrarenal lupus, and no extrarenal manifestations are documented in the other two patients. Data on SLE disease activity pre- and post- belatacept were available and scored in three of five patients using the SLEDAI-2KG which accounts for clinical and laboratory manifestations, as well as steroid use (Fig. 2). Mean ds-DNA pre and post belatacept was 133 ± 178 UI/mL and 58 ± 72 UI/mL, mean C3 89 ± 31mg/dL pre and 91 ± 21 mg/dL post, and mean C4 35 ± 15 mg/dL and 38 ± 7 mg/dL, respectively.
Conclusion: Belatacept in LN kidney transplant recipients may decrease extrarenal manifestations, attenuate CNI toxicity and stabilize allograft function, providing a better alternative to CNI regimens. Furthermore, these data suggest that belatacept may be a therapeutic option in SLE.
To cite this abstract in AMA style:
Carrión-Barberà I, Fajardo M, Tsapepas D, Guo C, Gartshteyn Y, Fernandez H, Askanase A. Belatacept in Systemic Lupus Erythematosus (SLE) Kidney Transplant Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/belatacept-in-systemic-lupus-erythematosus-sle-kidney-transplant-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/belatacept-in-systemic-lupus-erythematosus-sle-kidney-transplant-patients/