Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Belimumab, an inhibitor of B lymphocyte stimulator, is approved in adults with active, autoantibody-positive systemic lupus erythematosus (SLE) receiving standard of care.
Methods: Retrospective analysis was conducted in the Truven Health MarketScan® Commercial Claims and Encounters database (Sept 01, 2010 to Dec 31, 2015) (study 206345). Patients 18–64 years of age at first belimumab infusion (index) with a SLE diagnosis (ICD-9: 710.0 or ICD-10: M32) and continuous enrollment of 6 months pre-index and ≥3 post-index were analyzed. Patients diagnosed with lupus nephritis were excluded. The 6-month pre-index period was analyzed to characterize patients initiating treatment with intravenous belimumab. A previously published disease activity algorithm, developed based on claims data, was used to estimate the frequency of mild, moderate or severe flare symptoms.1
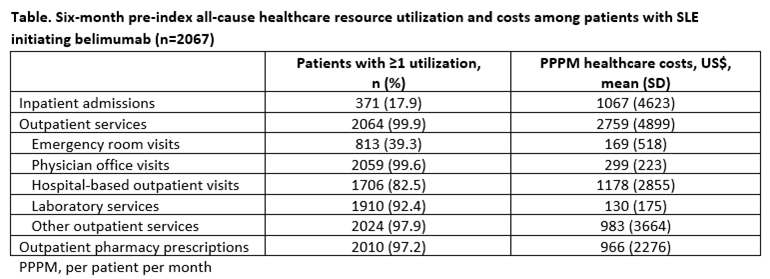
Results: This analysis comprised 2067 patients; 94.7% were female, mean (standard deviation [SD]) age was 43.9 (11.1) years and mean (SD) length of follow-up was 649.7 (434.8) days. In the 6-month pre-index period, mean (SD) Charlson Comorbidity Index score was 1.4 (0.9). Commonly observed comorbid conditions included hypertension (23.4%), myositis/myalgia (21.0%), and pulmonary disease (18.3%). The most frequent SLE-related clinical manifestations included arthralgia (28.4%) and hematologic disorders (20.8%). SLE‑related organ involvement included diseases of the musculoskeletal (98.5%), nervous (46.7%) and respiratory systems (40.5%).
Pre-index concomitant medications prescribed included corticosteroids (80.2%), antimalarials (66.0%), nonsteroidal anti-inflammatory drugs (38.2%), immunosuppressive agents (59.1%), and rituximab (1.1%). Mean (SD) daily prednisone-equivalent dose was 27.7 mg (87.5), with 12.7%, 25.7% and 40.9% receiving ≤7.5 mg/day, >7.5 to ≤15 mg/day and >15 mg/day, respectively. Patients had a mean (SD) of 2.9 (5.9) primary care visits and 4.2 (7.0) rheumatologist visits in the 6-month pre-index period (Table). The proportions experiencing a mild, moderate or severe flare pre-index were 43.9%, 91.2% and 12.7% (92.9% had a moderate/severe flare).
Conclusion: In this large sample of belimumab IV initiators in usual care settings, patients with SLE demonstrated substantial SLE-related clinical manifestations in the 6 months prior to initiating, with approximately 90% having evidence of a moderate/severe flare. Concomitant medications included many commonly prescribed therapies, with corticosteroids being the most frequently prescribed, and per patient monthly costs were high. Future studies will investigate whether belimumab treatment reduces healthcare utilization and costs.
Study funded/conducted by GSK. Editorial assistance provided by Jennie McLean, PhD, of Fishawack Indicia Ltd, UK, funded by GSK.
1Garris C et al. J Med Econ 2013;16:667–77
To cite this abstract in AMA style:
Bell CF, Priest J, Amelio J, Song X, Kan H, Stott-Miller M, Limone B, Noxon V, Costenbader KH. Baseline Characteristics of Patients Diagnosed with Systemic Lupus Erythematosus Initiating Treatment with Intravenous Belimumab [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/baseline-characteristics-of-patients-diagnosed-with-systemic-lupus-erythematosus-initiating-treatment-with-intravenous-belimumab/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-of-patients-diagnosed-with-systemic-lupus-erythematosus-initiating-treatment-with-intravenous-belimumab/