Session Information
Date: Sunday, November 13, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster II: Manifestations
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Clinically evident kidney disease eventually occurs in up to one-half of SLE patients. The aim of this study is to describe sociodemographic, clinical, serological and treatment characteristics of a multicenter and multiethnic Latin American SLE cohort (GLADEL 2.0. Grupo Latino Americano De Estudio del Lupus) of patients with or without lupus nephritis (LN).
Methods: The GLADEL 2.0 is an observational prevalent and incident cohort initiated in 2019. Forty-four centers from 10 Latin-American countries enrolled patients ≥18 years who fulfilled 1982/1997 American College of Rheumatology (ACR) classification criteria for SLE. Patients of this cohort were categorized into four different subsets: Group I: Without renal involvement (never); Group II: With prevalent renal involvement. currently inactive; Group III: With prevalent renal involvement. currently active. and Group IV: With incident renal (onset < 3 months) involvement with renal biopsy. Demographic, clinical manifestations, treatments, disease activity (SLEDAI- 2k) and SLICC/ACR Damage Index (SDI) were examined at baseline. A descriptive cross-sectional analysis of data collected from May 2019 to May 2022 was performed. Numeric variables are reported as medians (interquartile ranges IQR) and compared using Kruskall-Wallis test; categorical variables are reported as frequencies (percentages) and compared using Chi-square or Fisher tests, as appropriate.
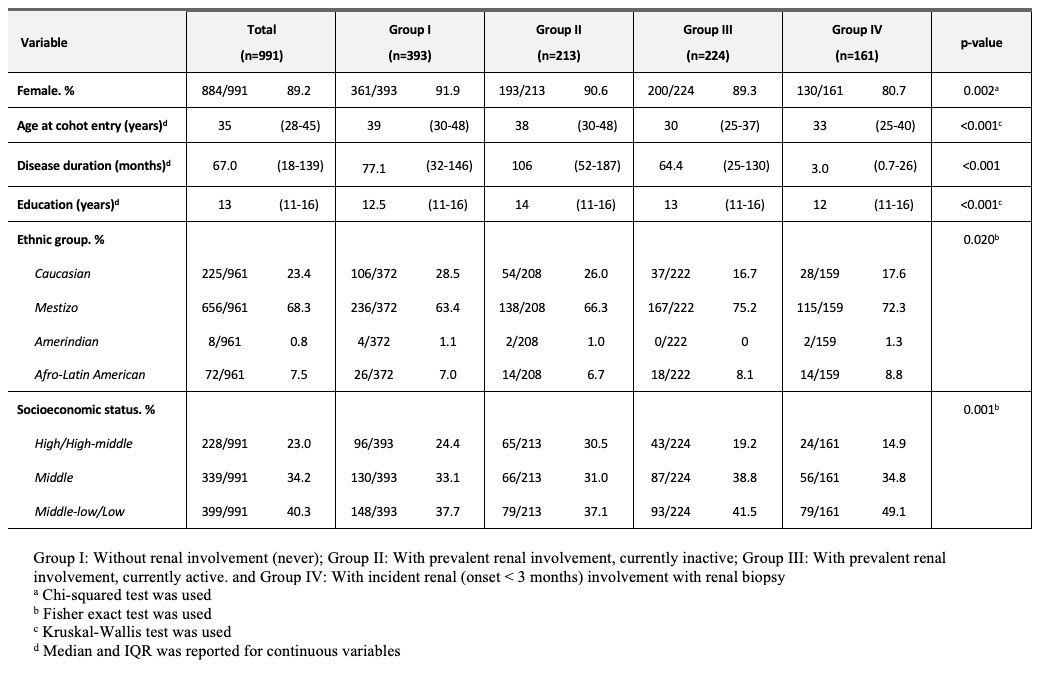
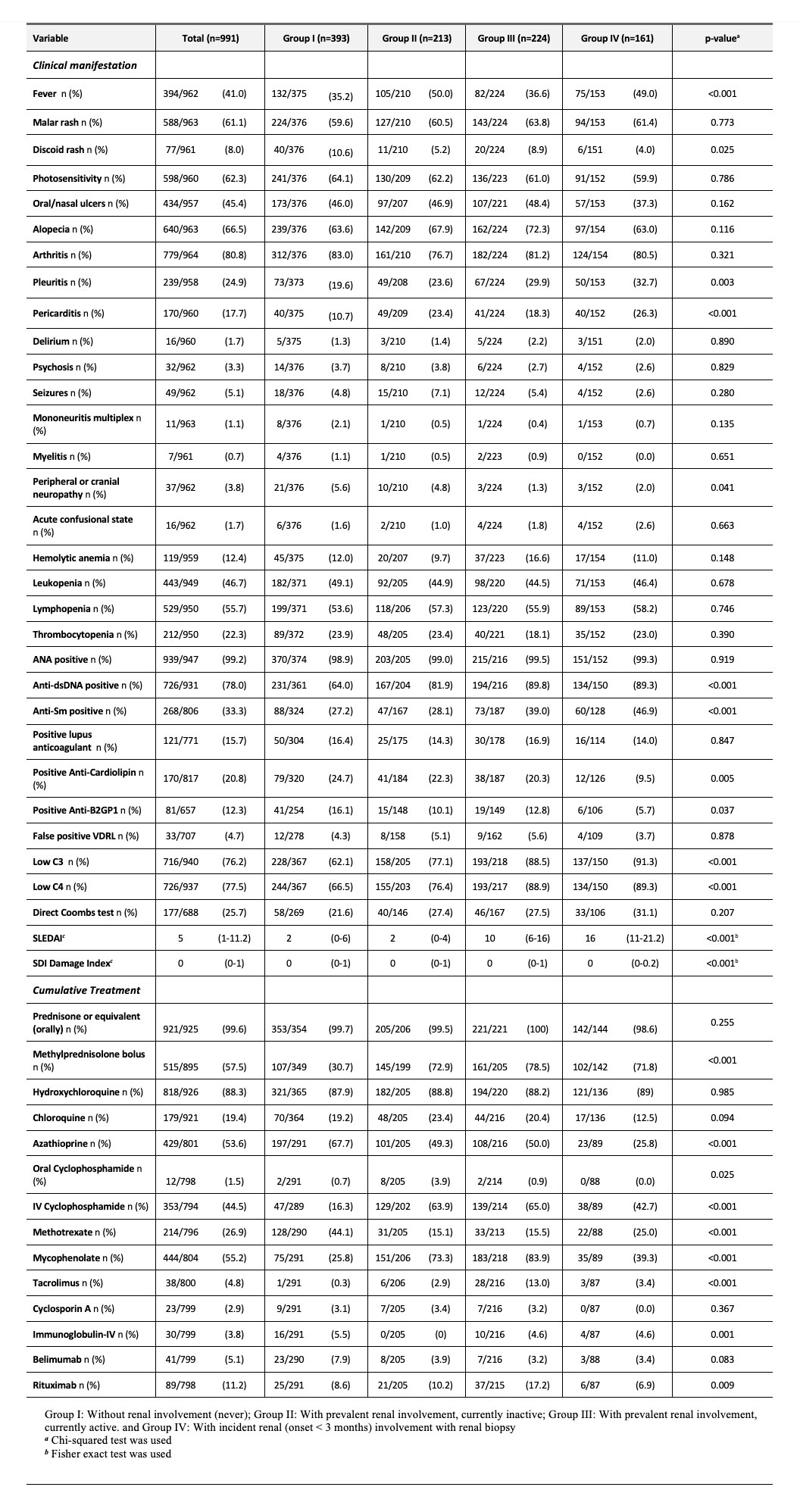
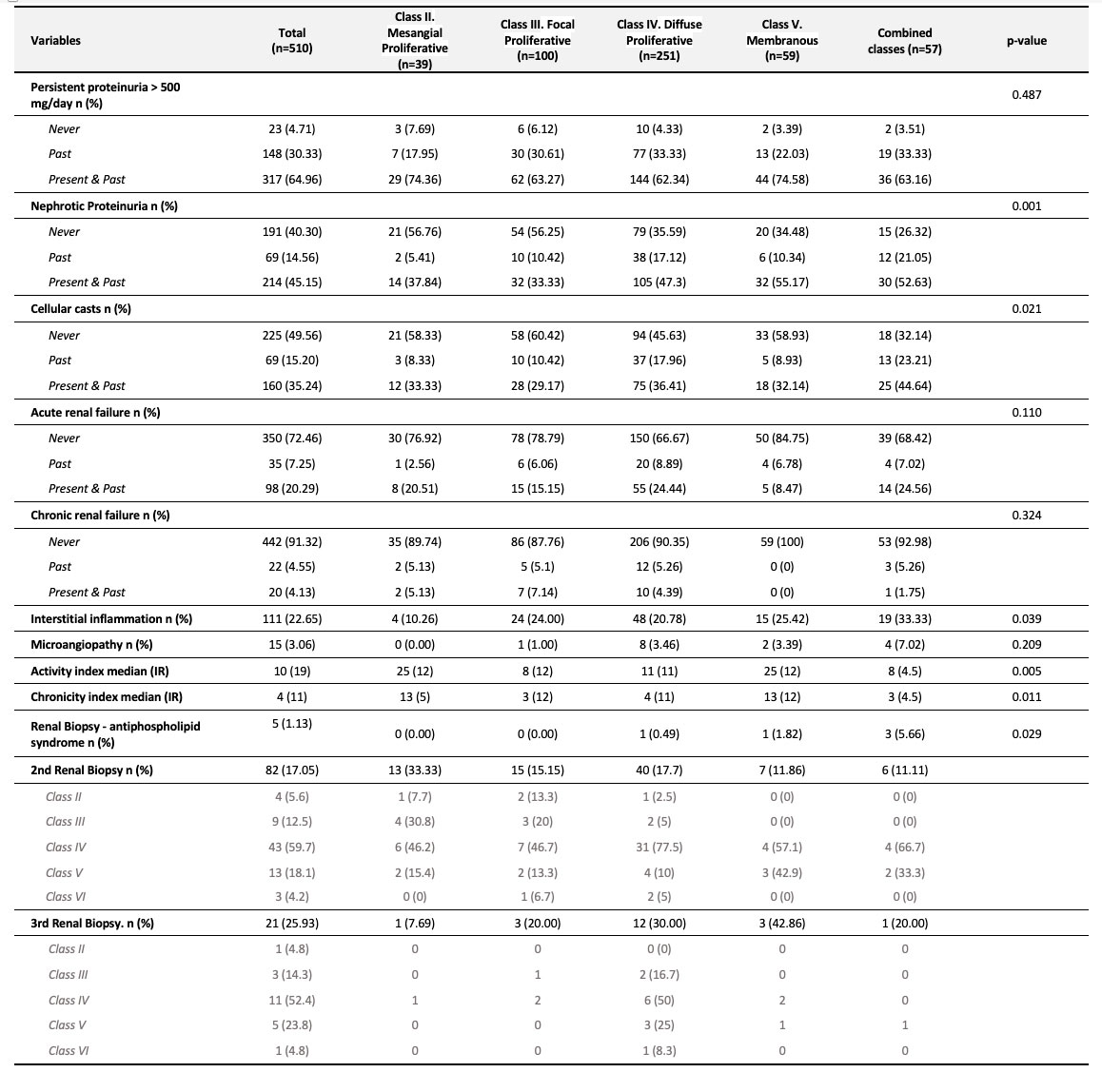
Results: A total of 991 SLE patients were included. 884 (89.2%) were female. Median (IQR) age at cohort entry was 35 (28-45) years. Median disease duration was 67 months (18-139) and 656 (68.3%) were Mestizos (of Amerindian and European ancestry). Patients with incident LN (group IV) had a higher proportion of males, were younger at diagnosis, had a shorter disease duration and were more frequently Mestizos (Table 1). Clinical, serological, treatment, disease activity and damage among groups are noted in Table 2, and revealed that for group I pericarditis and anti-dsDNA were less frequent and MMF was less often used by group I and IV. Table 3 shows the renal characteristics of the 510 patients (349 patients with prevalent LN from group II and III and 161 incident LN patients from Group IV) who underwent a kidney biopsy with a clear predominance of Class IV.
Conclusion: Baseline characteristics of the GLADEL 2.0 well characterized lupus patients’ cohort with or without LN are described with distinct demographic, clinical and laboratory patterns that will allow both centralized laboratory evaluation of urinary biomarkers and exploratory biomarker analyses including transcriptome and their impact on the outcome of these patients.
To cite this abstract in AMA style:
Nieto R, Quintana R, Borba e, Hernandez L, Fernandez-Avila D, Maurelli L, Alba P, Bordon F, Arizpe F, Berbotta G, Serrano-Morales R, Bertolaccini M, Kerzberg E, Angeles Gargiulo M, Rodriguez A, Barbosa V, gasparin A, Cavalcanti F, Alves Alvino L, Parente Costa Seguro L, Victoria de Oliveira Martins L, Niera o, Massardo L, Aroca Martinez G, Nieto Aristizabal I, Mendez Patarroyo P, iglesias Gamarra A, Zuniga Vera A, Vera-Lastra O, Perez Cristobal M, Martin-Nares E, Amezcua-Guerra L, Gonzalez-Bello Y, Gonzalez Enriquez O, Galarzo-Delgado D, Vazquez C, barrios M, Alba Linares M, Reategui C, Quiroz-Alva A, Polanco Mora T, Pizzarossa C, Rebella M, Crespo M, Danza A, Silva Dutra de Oliveira Bonfa E, Alarcón G, Zazzetti F, Orillion A, Pons-Estel G, Sbarigia U. Baseline Characteristics of a Longitudinal, Multinational, Multiethnic Study of Lupus Patients, with or Without Lupus Nephritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/baseline-characteristics-of-a-longitudinal-multinational-multiethnic-study-of-lupus-patients-with-or-without-lupus-nephritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-of-a-longitudinal-multinational-multiethnic-study-of-lupus-patients-with-or-without-lupus-nephritis/