Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
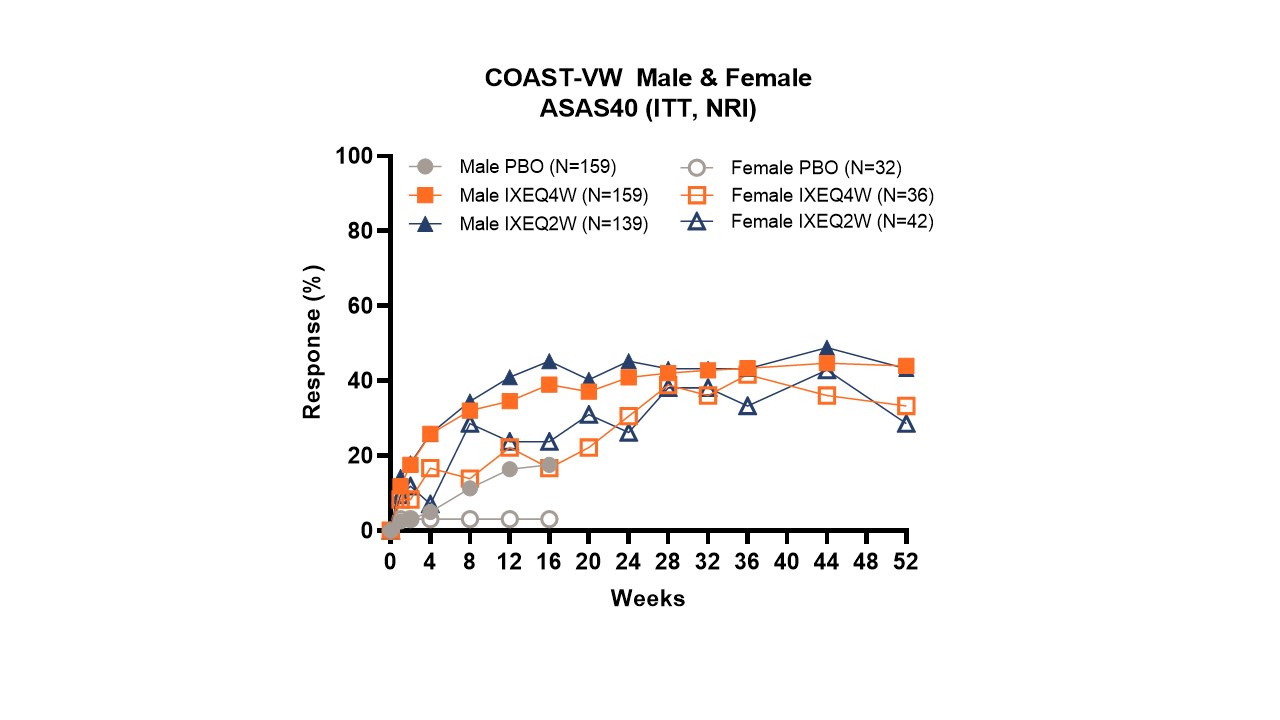
Background/Purpose: Axial spondyloarthritis (axSpA) is a chronic inflammatory disease of the axial skeleton comprising two subtypes within the same spectrum: radiographic (r-axSpA) and non-radiographic (nr-axSpA). Previous studies have shown that clinical presentation and treatment response of males and females may differ1 despite similar disease burden.2 Ixekizumab (IXE), a high-affinity monoclonal antibody that selectively targets interleukin-17A, has demonstrated superior efficacy to placebo in the treatment of patients with r-axSpA (COAST-V/W [bDMARD- naïve/TNFi-experienced]) and nr-axSpA (COAST-X [bDMARD-naïve]).3 Here we report baseline characteristics and treatment response to IXE categorised by sex in patients with r-axSpA and nr-axSpA for up to 52 weeks.
Methods: Patients fulfilled the ASAS classification criteria for r-axSpA or nr-axSpA. Patients were randomized to receive 80 mg subcutaneous IXE every 2 weeks (Q2W) or 4 weeks (Q4W), or to placebo (PBO) [16 weeks COAST-V/W; 52 weeks COAST-X]. Baseline characteristics and treatment outcomes were assessed. Patients were categorised by sex, missing data was controlled for using non-responder imputation (NRI) and modified baseline observation carried forward (mBOCF) analysis was conducted on continuous efficacy variables.
Results: At baseline, females were older, with significantly higher pain and fatigue scores and peripheral joint symptoms (table 1). ASAS40 response rate with IXEQ4W was achieved in 39% of males with r-axSpA by week 16, and 44% by week 52. Females achieved 16.7% at week 16, and 33.3% at week 52. In nr-axSpA, 46% of IXEQ4W males achieved ASAS40 at week 16 and 30% at week 52. 23.9% of females achieved ASAS40 at week 16, increasing to 30.4% at week 52.
Conclusion: This analysis demonstrates that for the axSpA disease spectrum, females present with higher disease burden as reflected by higher scores in fatigue/tiredness, and spinal pain at night. Our findings indicate that males and females respond to IXE; however, females experience this benefit later in their treatment course, with a more prolonged attainment of peak response.
References.
1. van der Horst-Bruinsma IE, et al. Ann Rheum Dis. 2019;78:1550-1558.
2. Zhao SS, et al. Rheumatology. 2019;58:2025-2030.
3. Deodhar A, et al. Lancet. 2020;395:53-64.
To cite this abstract in AMA style:
van der Horst-Bruinsma I, Bolce R, Hunter T, Sandoval Calderon D, Zhu D, Geneus V, Lisse J, Liu-Leage S, Magrey M. Baseline Characteristics and Treatment Response to Ixekizumab Categorised by Sex in Radiographic and Non-Radiographic Axial Spondylarthritis Patients Through 52 Weeks: Data from 3 Phase III, Randomized, Controlled Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/baseline-characteristics-and-treatment-response-to-ixekizumab-categorised-by-sex-in-radiographic-and-non-radiographic-axial-spondylarthritis-patients-through-52-weeks-data-from-3-phase-iii-randomize/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-and-treatment-response-to-ixekizumab-categorised-by-sex-in-radiographic-and-non-radiographic-axial-spondylarthritis-patients-through-52-weeks-data-from-3-phase-iii-randomize/