Session Information
Date: Tuesday, November 10, 2015
Title: Rheumatoid Arthritis - Human Etiology and Pathogenesis Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
The importance of type I interferons (IFN-α and others)
as a driver of pathogenesis in autoimmunity, including rheumatoid arthritis
(RA) is well recognized. However, in this context, the contribution of
type II interferons (IFN-γ) has been ambiguous. The
interferon gamma receptor (IFNGR) is a
heterodimer of proteins encoded by IFNGR1 and IFNGR2.
The expression of IFNGR2, not plasma levels of INF-γ, has been shown to be the limiting factor for
receptor binding. We recently reported a strong association between the
presence of RA and the level of expression of the INF-γ receptor gene IFNGR1 in peripheral blood
mononuclear cells (PBMC). We also analyzed 576 RA
patients and found that IFNGR2 in PBMC strongly associated with
radiographic severity (P=0.03 for
erosions, P=0.04 for joint space narrowing, and P=0.03 for total radiographic
score). These data implicate and
important pathogenetic function of the IFN-γ signaling pathway in RA.
 Methods: We
Methods: We
sought to determine the mechanistic significance of the elevated expression of INF-γ receptors within subpopulations of CD4 and CD8 T
cells, B cells, and monocytes. To address this question, we used a
phosphoflow approach (high-definition multi parameter flow cytometry with
simultaneous evaluation of activation of multiple STAT signaling molecules). We
analyzed STAT1, STAT3, and STAT5 cell signaling in na•ve and memory CD4+ and
CD8+ T cells (differentiated by CD45RA and CCR7), Tfh cells (CD4+/CXCR5-),
na•ve and memory B cells, and monocytes (CD11b+) in PBMC of RA (n=25) and
healthy controls (HC) (n=10).
Results:
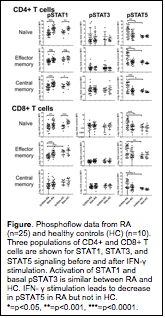
Although STAT1 is the primary STAT
activated following stimulation with INF-γ,
we found no difference in the activation of STAT1 between RA and HC in all cell
populations. However, we found that basal active STAT5 (as assessed by
phosphorylated tyrosine at position 694 [pY694-STAT5]) was elevated in RA compared
to HC (p=<0.05). Strikingly, this active basal pSTAT5 was
down-modulated following stimulation with INF-γ
in CD4 and CD8 T cell populations but not in B cell populations or monocytes
from RA (see figure).
Conclusion: Our novel preliminary findings suggest that decreased levels
of pSTAT5 following stimulation of T cell populations and Tfh cells with INF-γ contribute to the pathogenesis of RA and its
severity. Ongoing studies reveal that this down-modulation of STAT5 may
render cells hyporesponsive to regulatory cytokines such as IL-2.
To cite this abstract in AMA style:
Boland M, Ding Y, Vinod S, Bridges SL Jr., Raman C. Basal STAT5 Signaling Is Elevated in Multiple Peripheral Blood Cell Subsets in Rheumatoid Arthritis and Is Markedly Downregulated By IFN-γ in T Lymphocytes [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/basal-stat5-signaling-is-elevated-in-multiple-peripheral-blood-cell-subsets-in-rheumatoid-arthritis-and-is-markedly-downregulated-by-ifn-i%c2%b3-in-t-lymphocytes/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/basal-stat5-signaling-is-elevated-in-multiple-peripheral-blood-cell-subsets-in-rheumatoid-arthritis-and-is-markedly-downregulated-by-ifn-i%c2%b3-in-t-lymphocytes/
