Session Information
Date: Sunday, November 12, 2023
Title: (0176–0195) Healthcare Disparities in Rheumatology Poster I: Lupus
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite greater prevalence of lupus among diverse, racial and ethnic minority populations, marked gaps exist between populations affected by lupus and those enrolled in clinical trials. Sponsored by the Lupus Research Alliance, the Lupus Clinical Investigators Network (LuCIN) is the largest lupus clinical trials network in North America, comprised of 57 leading academic institutional sites across the US and Canada. This study aimed to describe and discuss prominent barriers and potential solutions to support recruitment and participation in lupus clinical trials conducted through the LuCIN network.
Methods: Data from the LuCIN annual survey of site investigators and research staff (site representatives) collected between March and July 2022 were prepared for analysis. The survey included questions to assess perspectives and practices around clinical trial recruitment. Descriptive statistics were computed to identify the most common barriers and facilitators to recruitment and retention of patients in lupus clinical trials.
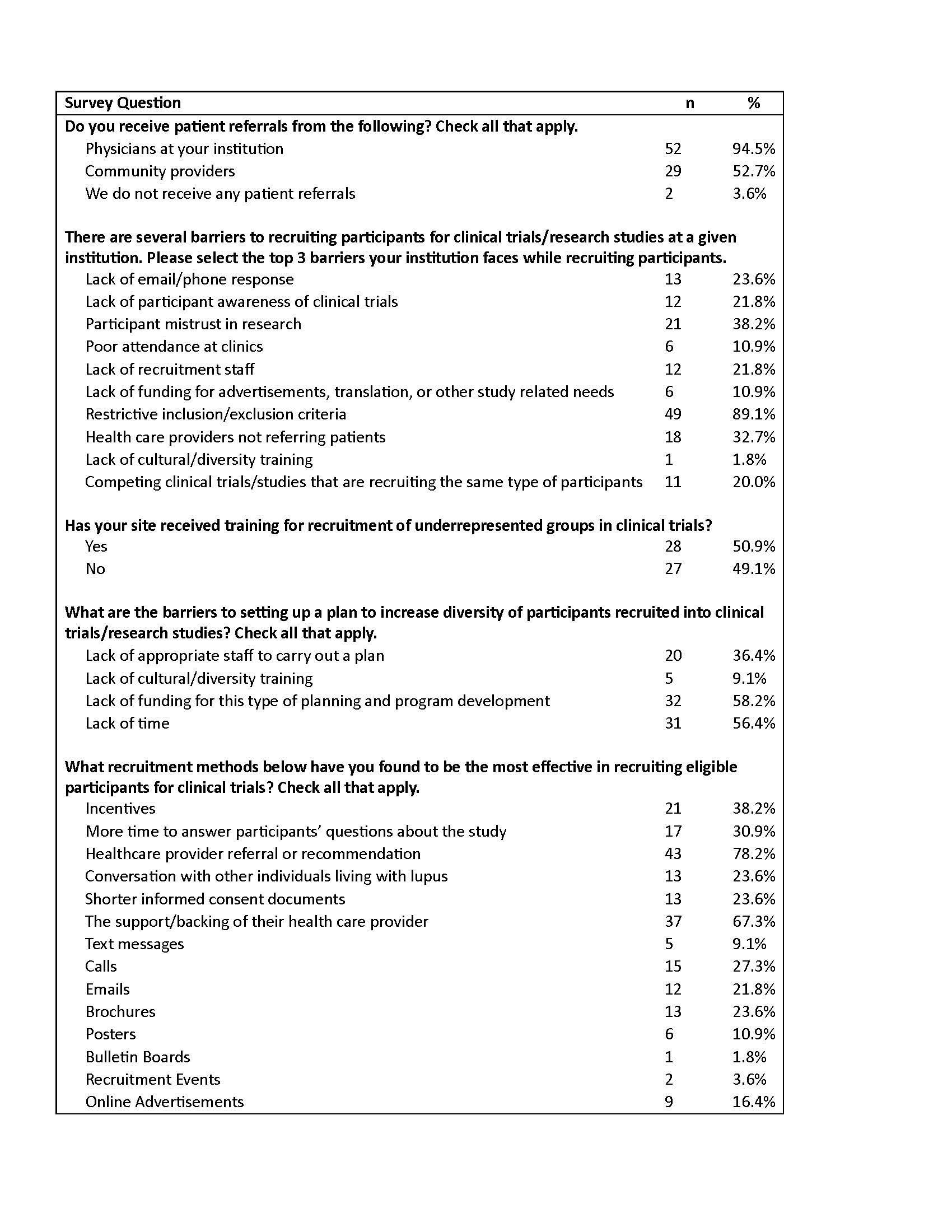
Results: Completed survey responses were collected from representatives of 55 LuCIN sites. When asked about sources of patient referrals at their site, most respondents reported receiving patient referrals to LuCIN clinical trials from physicians within their institution (94.5%), while referrals from external community clinicians were reported by 52.7% of respondents. The most frequently reported barriers to LuCIN clinical trial recruitment were restrictive inclusion/exclusion criteria (89.1%), participant mistrust in research (38.2%), and a lack of referrals from healthcare providers (32.7%). Approximately half (49.1%) of sites reported that they had not received training on recruitment of underrepresented patients in clinical trials. When asked about the barriers to establishing a plan to improve recruitment of diverse participants, over half of site respondents identified a lack of funding (58.2%) and time (56.4%) as barriers. The patient recruitment strategies respondents most frequently identified as effective approaches included healthcare provider referrals or recommendation (78.2%), support/endorsement from patients’ healthcare providers (67.3%), study incentives (38.2%), and additional time to answer potential participants’ study-related questions (30.9%).
Conclusion: This work provides valuable insights into key barriers and facilitators reported by sites to the recruitment of underrepresented participants into lupus clinical trials conducted through LuCIN. Findings underscore the need to support opportunities for effective provider-patient communication, as well as engagement between academic and community practices, to improve access and referrals to lupus clinical trials. Future research is needed to explore how these barriers and facilitators relate to clinical trial participation outcomes among underrepresented patients. By understanding these challenges and leveraging successful recruitment strategies, LuCIN clinical trial sites can promote equity in clinical trial participation among underrepresented groups and further advance lupus research.
To cite this abstract in AMA style:
Englund T, Lee C, Hsieh J, Araojo R, Mariano J, McCormick E, Bell S, Roy A, Sheikh S. Barriers and Facilitators to Recruiting Underrepresented Participants for Clinical Trials: Insights from the Lupus Clinical Investigators Network (LuCIN) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/barriers-and-facilitators-to-recruiting-underrepresented-participants-for-clinical-trials-insights-from-the-lupus-clinical-investigators-network-lucin/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/barriers-and-facilitators-to-recruiting-underrepresented-participants-for-clinical-trials-insights-from-the-lupus-clinical-investigators-network-lucin/