Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In Phase 3 trial, RA-BEAM, baricitinib (BARI), a JAK1/JAK2 inhibitor, was associated with significant clinical improvements vs. placebo (PBO) and adalimumab (ADA) in RA patients.1 Although BARI and ADA had similar improvement in swollen joint count, BARI demonstrated statistically significantly greater pain improvement. While pain is a generic feature of inflammation, not all pain in RA is due to inflammation, and the contribution of different pathways to pain is unclear. In this post hoc analysis, we assessed the relationship between pain improvement and disease activity and evaluated whether BARI provided additional pain improvement vs. PBO and ADA across levels of disease activity.
Methods: 1,305 patients on stable background MTX were randomized 3:2:3 to and treated with PBO, 40 mg subcutaneous ADA every other week, or daily, oral 4 mg BARI. Pain was assessed with a 0-100 mm visual analog scale (VAS); pain improvement from baseline to Week 12 was grouped by ≤30%, >30% to ≤50%, >50% to ≤70%, and >70%2. Disease activity was measured with the Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), Disease Activity Score for 28 joints (DAS28) with C-reactive protein (CRP), and DAS28 with erythrocyte sedimentation rate (ESR). To evaluate change in pain with disease activity, regression was used with continuous change in pain VAS score from baseline to Week 12 as the outcome and continuous CDAI/SDAI/DAS28-CRP/DAS28-ESR values, treatment, and the interaction term between treatment and disease activity as explanatory variables. Last observation carried forward was used to impute missing values. Pain VAS change at Week 12 was estimated from regression for all treatments if patients achieved remission (REM)/low disease activity (LDA)/moderate disease activity (MDA) as defined by the corresponding clinical measure. Analyses were not adjusted for multiplicity. Data visualization with the percentage of pain VAS improvement vs. disease activity was created to examine pain improvement with treatment over time.
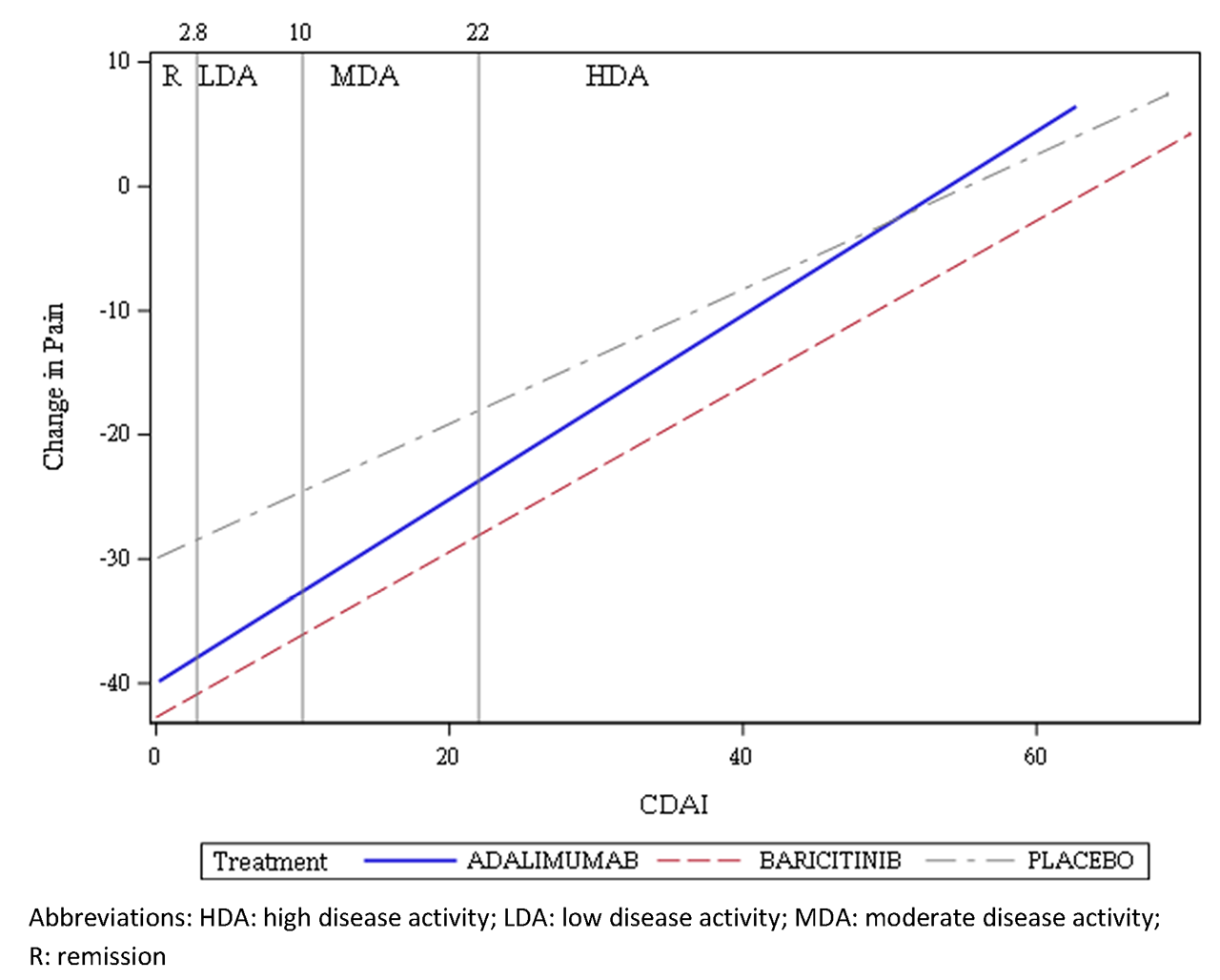
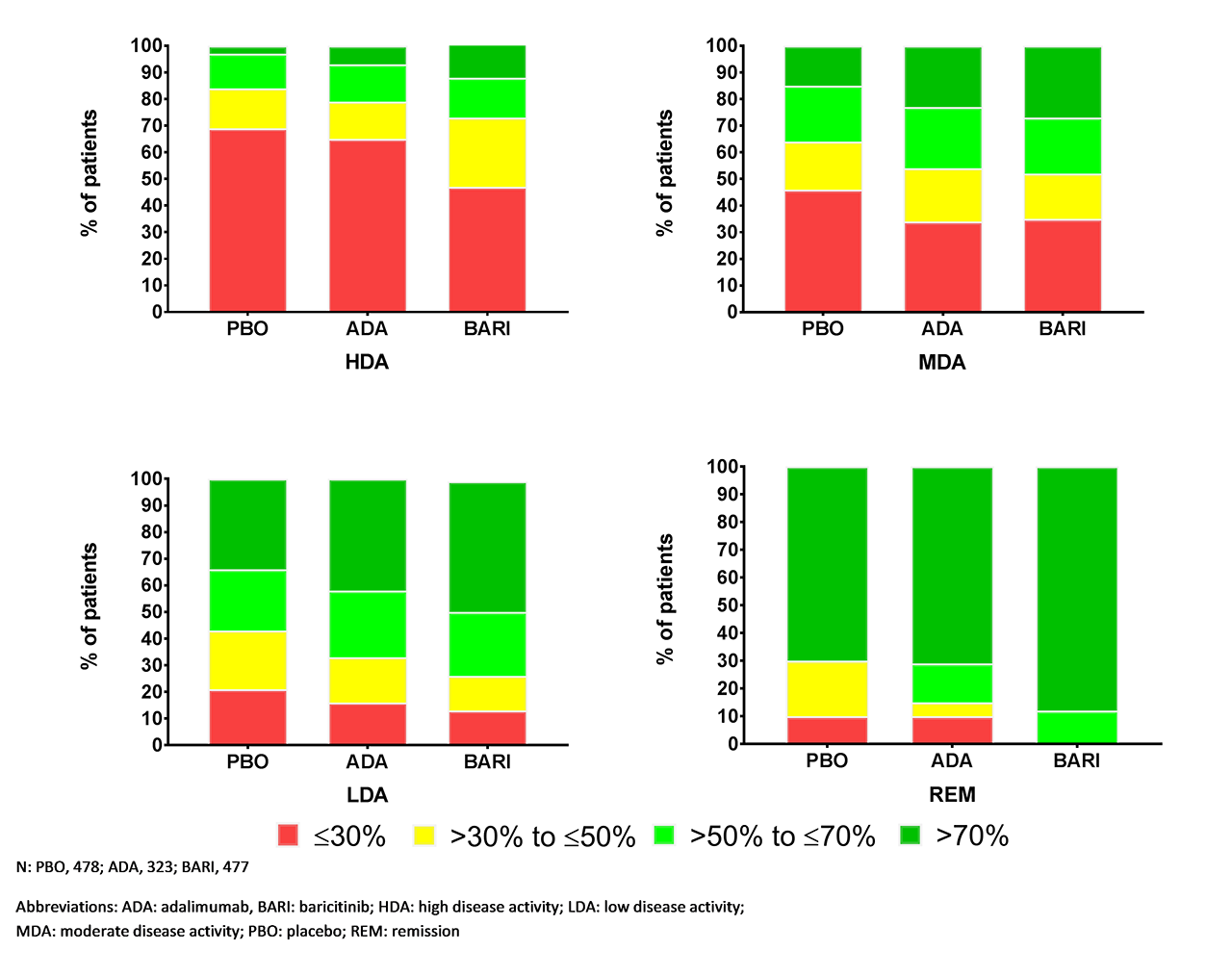
Results: With CDAI, 91% of patients had high disease activity and 9% had MDA across all treatments at baseline. At Week 12, the percentage of patients who achieved REM with PBO, ADA, and BARI, respectively, were 2%, 7%, 8%; for LDA: 15%, 27%, 33%; for MDA: 33%, 40%, 38%.3 At all CDAI values, the estimated change in pain VAS for BARI was greater vs. PBO and ADA (Figure 1); the BARI line is below lines for PBO and ADA across the CDAI range. Similar trends were observed with other disease activity measures (Table). Figure 2 shows the percentage of patients with ≤30%, >30% to ≤50%, >50% to ≤70%, and >70% pain improvement from baseline at Week 12 by disease activity. BARI demonstrated greater pain improvement vs. ADA and PBO in all disease activity categories. With CDAI/SDAI, greater differentiation between BARI and ADA was observed as CDAI/SDAI values increased.
Conclusion: BARI provided additional pain improvement vs. PBO and ADA when disease activity was controlled and across all levels of disease activity, as measured by CDAI, SDAI, DAS28-CRP, or DAS28-ESR at Week 12.
References: 1. Taylor, NEJM, 2017; 2. http://www.immpact.org/; 3. Nash ACR abstract 2017
To cite this abstract in AMA style:
Taylor P, Pope J, Ikeda K, Zhang X, Jia B, Zhang H, Quebe A, Chen Y, Gaich C, Holzkaemper T, Cardoso A, Sebba A. Baricitinib Provides Better Pain Relief Across All Disease Activity Levels Compared with Placebo and Adalimumab in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/baricitinib-provides-better-pain-relief-across-all-disease-activity-levels-compared-with-placebo-and-adalimumab-in-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-provides-better-pain-relief-across-all-disease-activity-levels-compared-with-placebo-and-adalimumab-in-rheumatoid-arthritis/