Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Baricitinib (Bari), an oral selective inhibitor of Janus kinase (JAK)1 and JAK2, has been approved for the treatment of RA in the Europe and Japan. The purpose was to report results from a 24-week (wk) global, Phase 2, double-blind, placebo (PBO)-controlled study of Bari in patients with SLE receiving standard therapy.
Methods: Patients with SLE (positive ANA or anti-dsDNA, clinical SLEDAI-2K ≥4, arthritis or rash required) receiving stable background SLE therapy were randomized 1:1:1 to PBO, or Bari (2- or 4-mg) once daily. The primary endpoint was resolution of SLEDAI-2K arthritis or rash at Wk24.
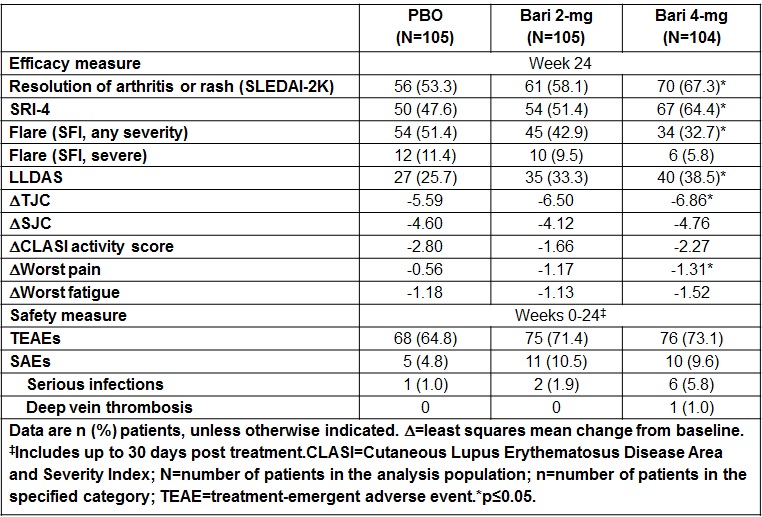
Results: Of 314 patients randomized, 79%, 82%, and 83% completed 24 wks of treatment in PBO, Bari 2-mg, and Bari 4-mg groups, respectively. At Wk24, a significantly greater proportion of patients in Bari 4-mg group compared to PBO achieved resolution of SLEDAI-2K arthritis or rash (67% vs 53%, p<0.05); and SLE Responder Index (SRI)-4 response (64% vs 48%, p<0.05). At Wk24, the proportion of patients achieving flare reduction (SELENA-SLEDAI Flare Index [SFI]), Lupus Low Disease Activity State (LLDAS), and tender joint count (TJC) change from baseline were also significantly improved for Bari 4-mg compared to PBO (Table). No statistically significant differences were observed between Bari 2-mg and PBO in any of the above endpoints. Rates of adverse events leading to treatment discontinuation and serious adverse events (SAEs) were higher for both Bari dose groups compared to PBO. There were no deaths, malignancies, major adverse cardiovascular events, tuberculosis, or serious herpes zoster infections; 1 SAE of deep vein thrombosis was reported in a patient with risk factors (Bari 4-mg group).
Conclusion: In patients with SLE receiving standard background therapy, once-daily oral Bari 4-mg was associated with significant clinical improvements compared to PBO and an acceptable benefit/risk profile. These findings support further study of Bari 4-mg as a potential therapy for patients with SLE.
To cite this abstract in AMA style:
Wallace DJ, Furie R, Tanaka Y, Kalunian KC, Mosca M, Petri M, Dorner T, Cardiel MH, Bruce IN, Gomez E, DeLozier AM, Janes J, Linnik MD, de Bono S, Silk ME, Hoffman RW. Baricitinib in Patients with Systemic Lupus Erythematosus: Results from a Phase 2, Randomized, Double-Blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/baricitinib-in-patients-with-systemic-lupus-erythematosus-results-from-a-phase-2-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-in-patients-with-systemic-lupus-erythematosus-results-from-a-phase-2-randomized-double-blind-placebo-controlled-study/