Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases I
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Moderate glucocorticoids (GCs) improve nearly all cases of polymyalgia rheumatica (PMR) but related adverse events are common in senior patients. The purpose of this randomized placebo-controlled trial in early PMR was to evaluate whether baricitinib (JAK 1/2 inhibitor) allows to reach disease control without GCs in recent-onset PMR. Moderate glucocorticoids (GCs) improve nearly all cases of polymyalgia rheumatica (PMR) but related adverse events are common in senior patients. The purpose of this randomized placebo-controlled trial in early PMR was to evaluate whether baricitinib (JAK 1/2 inhibitor) allows to reach disease control without GCs in recent-onset PMR.
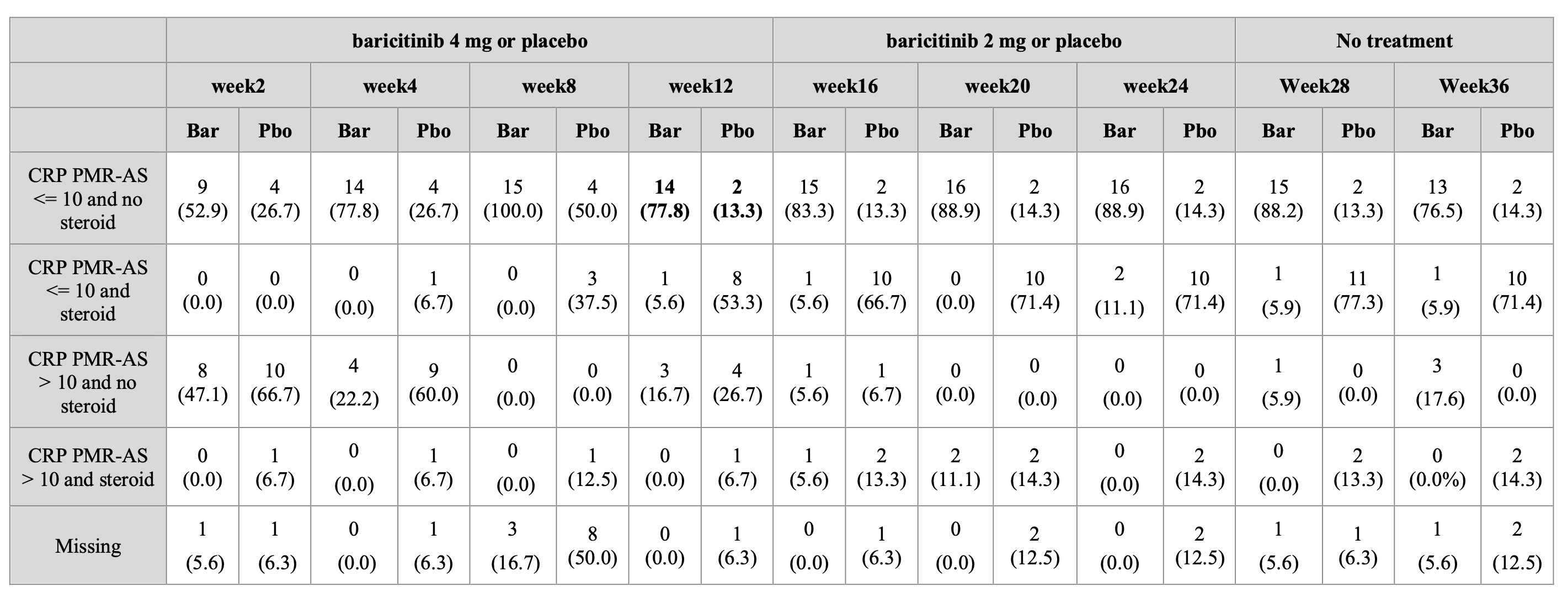
Methods: We conducted a double-blind randomized (1:1) placebo-controlled parallel-group trial between December 1, 2020, and August 30, 2023. Thirty-four patients with recent (< 6 months) naïve to GCs PMR with C Reactive PMR activity score (CRP PMR-AS) >17 received oral baricitinib 4 mg or matching placebo, with oral GCs rescue in cases of high disease activity for 12 weeks, and then baricitinib 2 mg or matching placebo, until week 24. Subdeltoid GC injection at W0 and W4 as permitted. The primary endpoint was the CRP PMR-AS≤10 without oral GCs rescue from W0 to week 12.
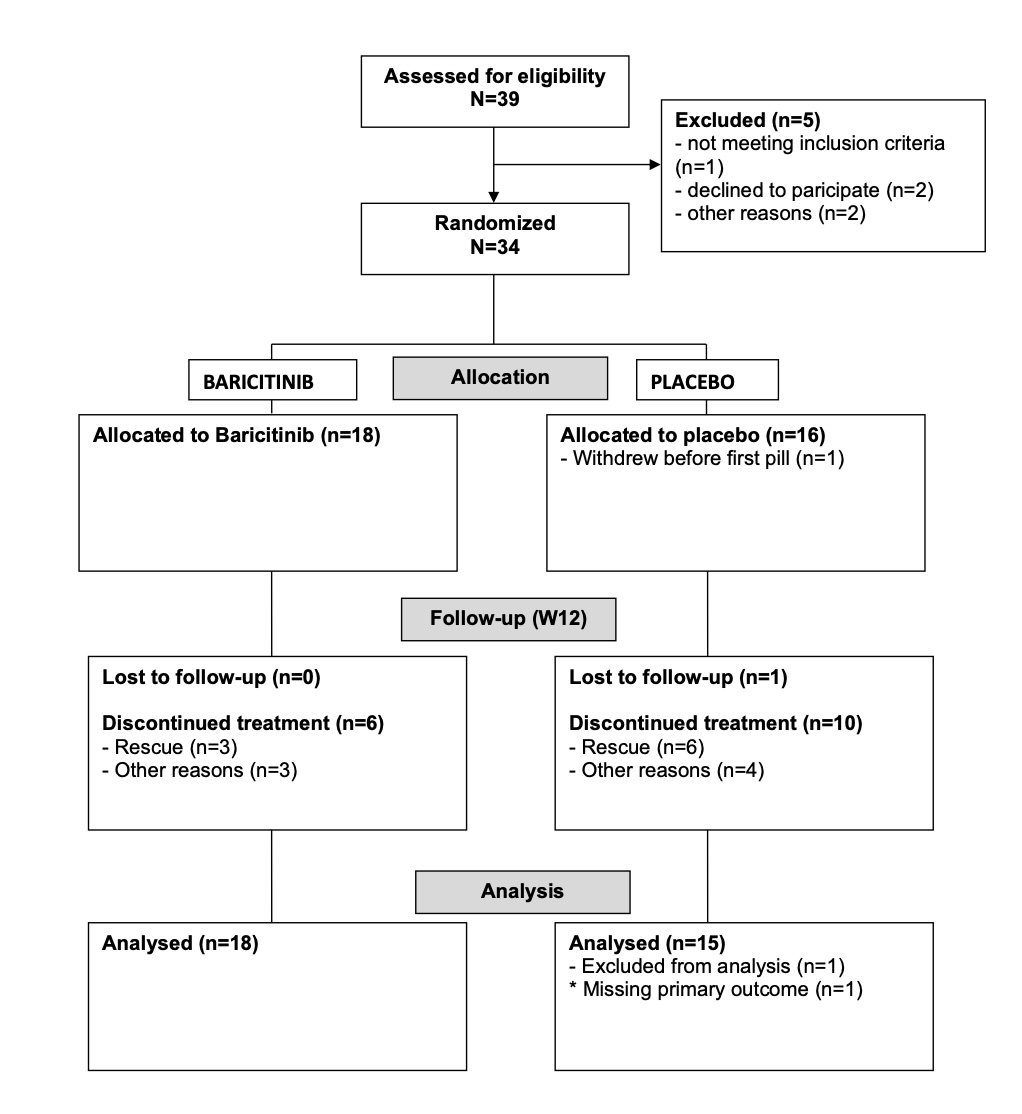
Results: We randomized 34 patients (18 baricitinib and 16 placebo) (Figure 1). The primary endpoint was reached at week 12 by 14/18 (77.8%) and 2/16 (13.3%) of patients in the baricitinib and placebo groups, respectively (RR (95% CI): 5.8 (3.2; 10.6); crude p value=0.0004; adjusted p value< 0.0001) (Figure 2). Among secondary outcomes assessed at week 12 erythrocyte sedimentation rate (ESR) PMR-AS, ESR and visual analogic score pain were significantly lower in the placebo group than in the baricitinib group (p=0.02, 0.02 and 0.03 respectively) but patients of the placebo group received more GCs and patients of the baricitinib group had a higher MCS SF36 and EQ5D (p=0.005 and 0.043, respectively). There was no flare until week 36. There was no death, no major adverse cardiovascular events or safety signal.
Conclusion: This study suggests that baricitinib alone 4 mg 12 weeks and then 2 mg 12 weeks allows sustained low disease activity 36 weeks in early PMR patients without safety signal.
To cite this abstract in AMA style:
SARAUX A, CARVAJAL ALEGRIA G, Dernis E, roux c, Richez C, TISON A, Quere B, Jousse-Joulin S, Guellec D, Marhadou T, Kervarrec P, Cornec D, Le Henaff C, Lesven S, emmanuel n, Souki A, Devauchelle V. Baricitinib in Early Polymyalgia Rheumatica (BACHELOR Study) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/baricitinib-in-early-polymyalgia-rheumatica-bachelor-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-in-early-polymyalgia-rheumatica-bachelor-study/