Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Baricitinib (bari) is an oral JAK1/JAK2 inhibitor approved for the treatment of moderately to severely active RA in adults in over 40 countries including European countries, the United States, and Japan. In the 52-week Phase 3 RA-BEAM study, bari 4 mg once daily (QD) showed clinical improvements compared to placebo (PBO) and to adalimumab (ADA) in MTX-inadequate-responder (IR) patients (pts).1 The objective of this analysis was to assess if pts who receive bari early attain added clinical improvement compared to pts with a delayed start of therapy.
Methods: In RA-BEAM 1305 pts (mean disease duration 8.7 years) were randomized 3:3:2 to PBO, bari-4 mg QD, or ADA 40 mg every 2 weeks. At Week 16 or subsequent visits, IRs (lack of ≥20% reduction in tender and swollen joint count) were rescued to open-label bari-4 mg. At Week 24 PBO pts were switched to bari-4 mg. Patients initially randomized to bari-4 mg were considered the early start group for this analysis and PBO pts switched at Week 24 or rescued at Week 16 or later were considered delayed start. Change from baseline using mixed model repeated measures and mean scores were assessed for the Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), DAS28-hsCRP, DAS28-ESR, HAQ-DI, and modified total Sharp score (mTSS) to compare the early vs delayed start groups between Weeks 24 and 52.
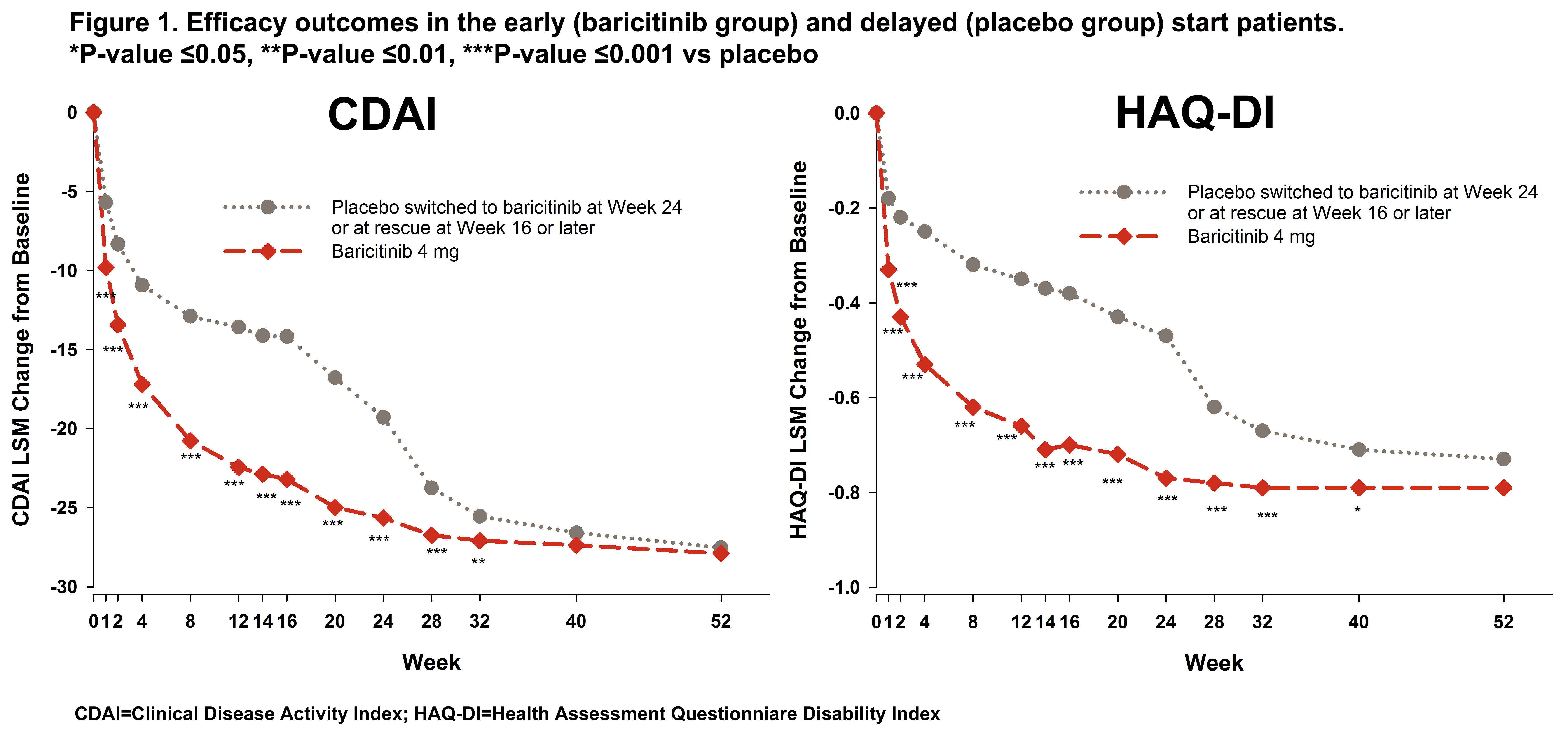
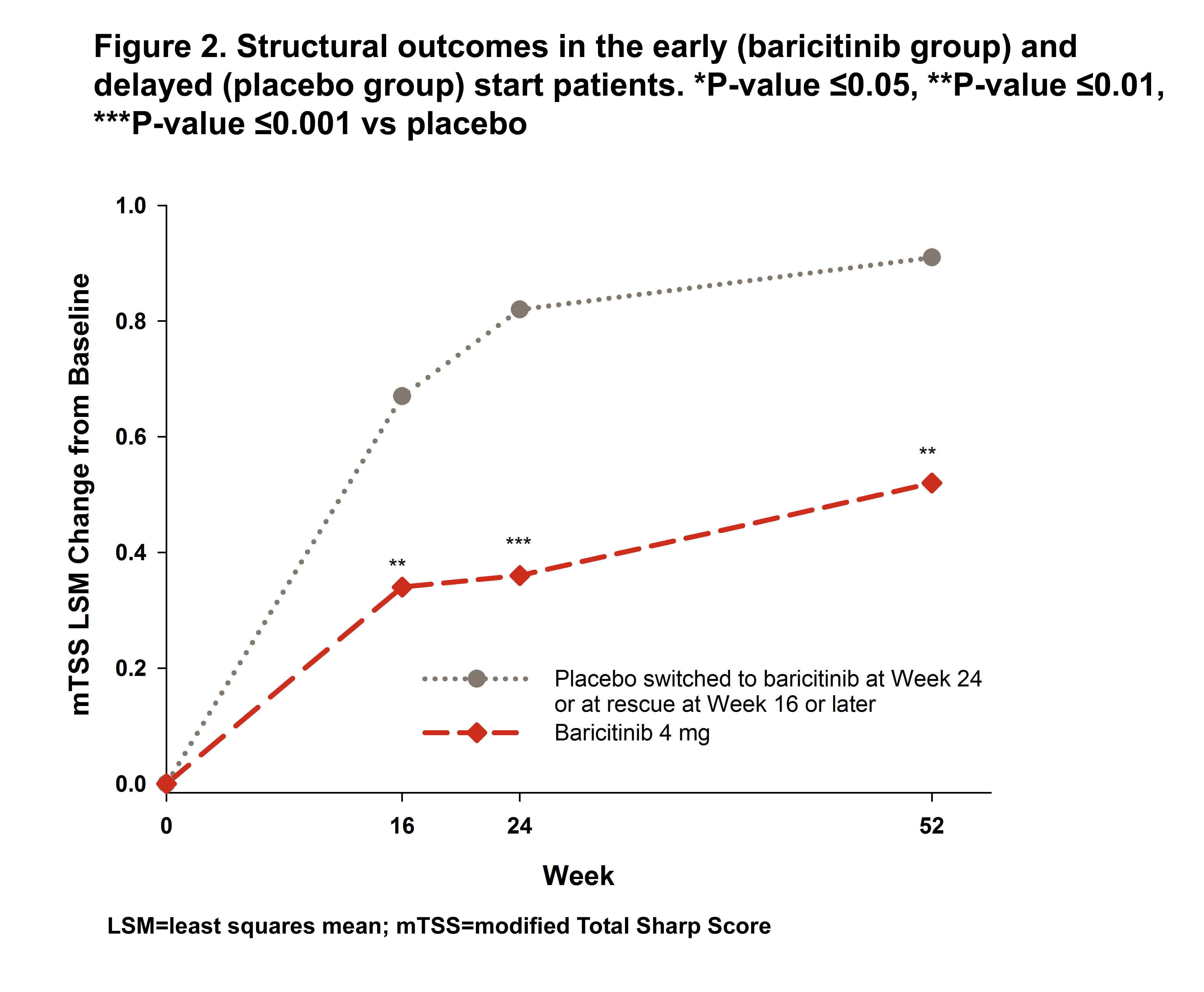
Results: In the early and delayed start groups, 35 (7.2%) and 128 (26.2%) pts were rescued to bari between Weeks 16 and 24, respectively. The early start group had significantly greater change from baseline up to Week 32 and showed greater and more rapid reduction through the first 4 weeks (>50% reduction) for CDAI compared to the delayed start group (Figure 1). At Week 24, the delayed start group showed a CDAI reduction similar to the reduction achieved by the early start group between Weeks 4 and 8 giving the early start group a 4 to 5 month advantage in disease improvement compared to the delayed start group. After receiving bari, the delayed start group also showed a rapid improvement in CDAI and caught up to the early start patients by Week 40. Similar results were seen for SDAI, DAS28-ESR, and DAS28-hsCRP. However, the early start pts had significantly greater improvement in HAQ-DI still maintained at Week 40 (Figure 1) and a significant advantage from Weeks 16 to 52 (Figure 2) for mTSS.
Conclusion: While overall disease activity improvement was similar, early start of bari treatment provided faster efficacy. A delay of up to 6 months in bari treatment had an impact on HAQ-DI and structural damage progression.
To cite this abstract in AMA style:
Taylor PC, Tanaka Y, Cardoso A, Zhong J, Chen YF, Workman JL, Morales LDC, Schiff M. Baricitinib: Early Vs. Delayed Start in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/baricitinib-early-vs-delayed-start-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-early-vs-delayed-start-in-patients-with-rheumatoid-arthritis/