Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Despite the wide use of AZA during pregnancy, there are no studies evaluating the impact of pregnancy on AZA metabolites 6-thioguanine nucleotide (6-TGN) and 6-methylmercaptopurine nucleotide (6-MMPN) disposition in rheumatic diseases. The purpose of this study was to characterize changes in AZA metabolite concentrations throughout pregnancy in women with rheumatic disease and explore the relationship between metabolite concentrations, maternal disease activity, and neonatal outcomes.
Methods: Patients with rheumatic disease prescribed AZA prior to pregnancy and at least one blood sample during pregnancy between 5/2016-4/2022 were included. Azathioprine metabolite concentrations were quantified using High Performance Liquid Chromatography/Tandem Mass Spectrometry assay. Mean 6-TGN and 6-MMPN concentrations in patients on stable dosages were analyzed by trimester using analysis of variance (ANOVA). The relationship between 6-TGN concentration and SLE physician global assessment (PGA) was assessed using repeated correlation measures. Pregnancy-average 6-TGN and gestational age at birth was analyzed using unadjusted linear regression.
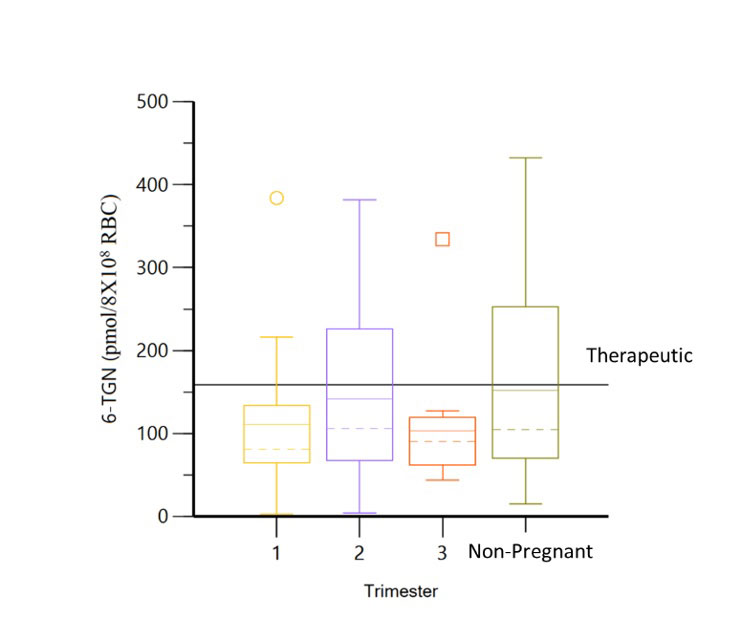
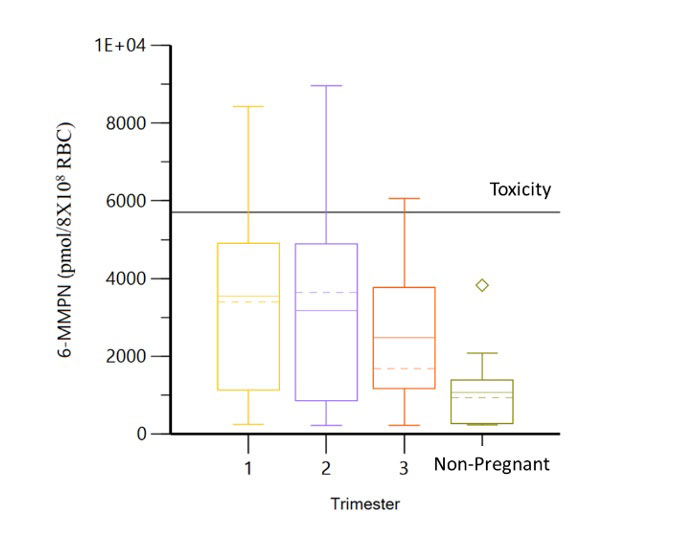
Results: Thirty-eight pregnancies in 36 women (table 1) with 110 serum samples were included. In patients without dose changes, there was no significant difference in 6-TGN concentrations across each trimester and prepartum/postpartum (p=0.6) (figure 1); whereas 6-MMPN concentrations were higher in early pregnancy (p=0.003) (figure 2). No elevated transaminases were observed with 6-MMPN >5700 pmol/8X108 RBC. Metabolite concentrations were related to total AZA dosage, weight-based dosage, and thiopurine methyltransferase phenotype. For women with SLE, there was no linear relationship between 6-TGN and SLE PGA scores (p=0.38), but there was a trend towards lower SLE PGA in those with therapeutic 6-TGN (≥ 159 pmol/8X108 RBC). There was a non-significant increase in gestational age at birth with pregnancy-averaged 6-TGN concentrations. Of 4 pregnancies with average 6-TGN in the therapeutic range, none delivered preterm.
Conclusion: We did not observe systematic changes in 6-TGN concentrations throughout pregnancy, but levels of 6-MMPN were higher in the first two trimesters without development of elevated transaminases, possibly due to higher body weight in late pregnancy and postpartum. Our results suggest that AZA dosing in pregnancy can be approached similarly to non-pregnant patients including the use of total body weight for initial dosage and lower dosages for patients with low or intermediate TPMT activity. In patients achieving therapeutic 6-TGN concentrations (≥ 159 pmol/8X108), there was a trend towards improved maternal disease activity and neonatal outcomes, but this did not reach statistical significance. Due to patient variability in AZA disposition during pregnancy, therapeutic drug monitoring may help individualize dosing and identify medication non-adherence. Additional studies are required to identify target AZA metabolite concentrations during pregnancy.
To cite this abstract in AMA style:
Balevic S, Sims C, Eudy A, Clowse M. Azathioprine Metabolite Levels and Outcomes During Pregnancies in Women with Rheumatic Disease [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/azathioprine-metabolite-levels-and-outcomes-during-pregnancies-in-women-with-rheumatic-disease/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/azathioprine-metabolite-levels-and-outcomes-during-pregnancies-in-women-with-rheumatic-disease/