Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Currently, histological assessment of synovial tissue (ST) in Rheumatoid Arthritis (RA) is hampered by 3 main factors: 1) tissue is a finite resource, 2) sequential sections of single conventional immunohistochemistry stains do not adequately preserve architectural and co-staining data, and, 3) image analysis and quantification is open to interpretation and human error. Furthermore,in RA, growing evidence implicates heterogeneous histopathological phenotypes with different disease outcomes and treatment responses, critical, as 30-40% of patients do not respond to current therapies and long-term drug-remission is rare, reflecting differential recruitment of inflammatory pathways and cell types. Thus, there is an unmet need for tools that can perform automated multi-parameter cellular identification and phenotype analysis on tissue sections, enabling translational research to assist is the development of personalised medicine.
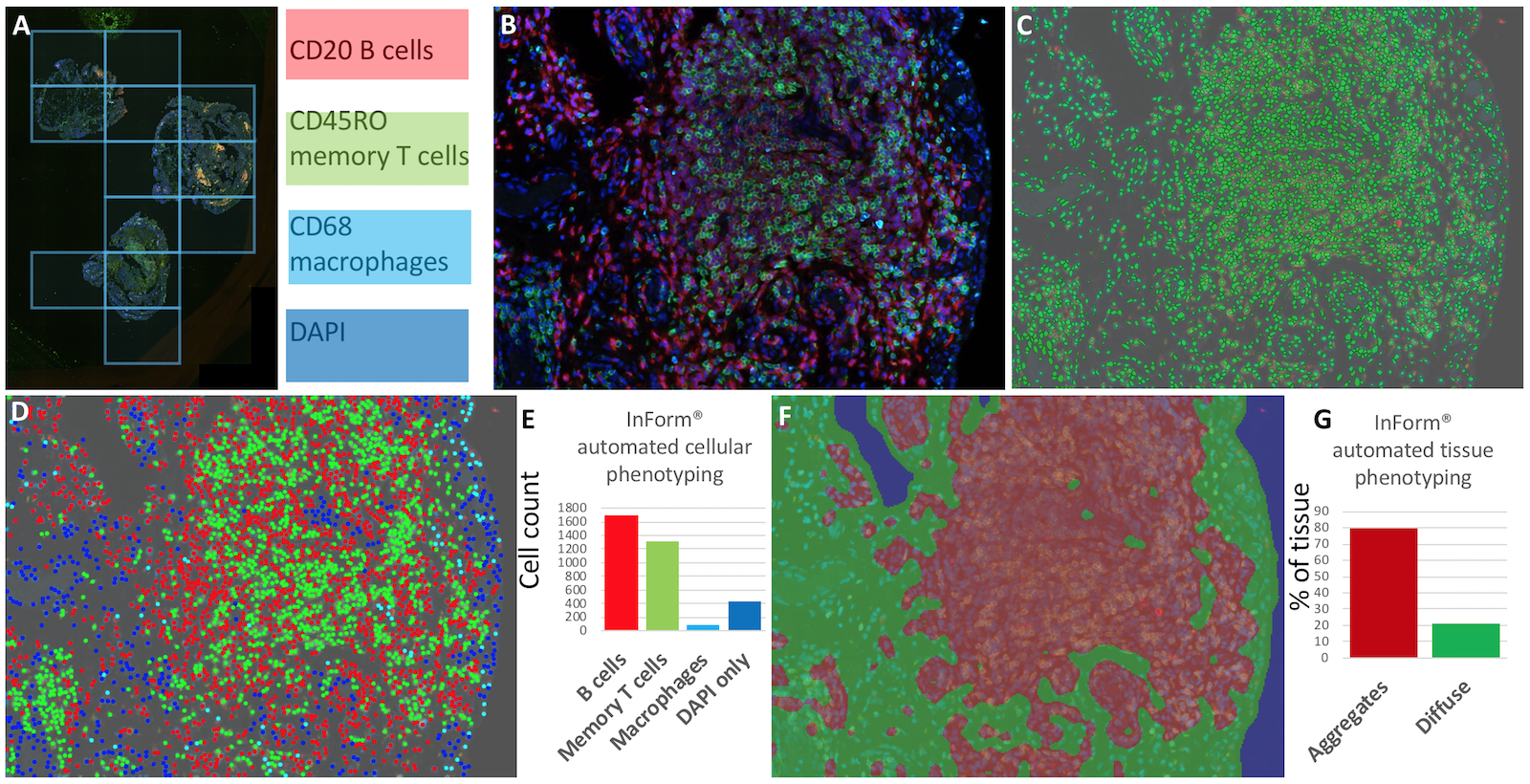
Methods: Sequential 4μm thick RA ST sections were prepared for Opal multispectral imaging. In brief, for Opal; primary antibodies identifying memory T (CD45RO), Β (CD20) and macrophage cell (CD68) are stained in turn, each followed by specific Opal reactive fluorophores. Primary antibody stripping prepares the section for the next primary antibody ready to repeat the process. Once all Opal targets have been developed, DAPI nuclear staining is performed prior to whole slide scanning (Figure 1A) and high-resolution (x20 magnification) image acquisition for CD45RO (green), CD20 (red), CD68 (cyan) and DAPI nuclear stain (blue) (Figure 1B) on the Perkin Elmer Vectra 3.0 automated imaging system.
Results: The image analysis and machine learning software package, inForm® was used to segment individual cells (Figure 1C), identify and quantify cellular phenotype (Figure 1D&E) and distinguish regions with “follicular” and “diffuse” staining patterns (Figure 1F&G).
Figure 1. Automated multiparameter imaging and analysis of RA ST. A, whole slide scanning. Β, high-resolution multiparameter microscopy for CD20 (red), CD45RO (green) and CD68 (cyan). C, cell segmentation map. D, cellular phenotyping and E, automated cellular counting. F, tissue phenotyping and G, automated phenotype assessment.
Conclusion: Due to the recent surge in interest in ST biology, in particular in clinical trials investigating biologic therapies, there is a need for effective, reproducible and high-throughout technologies for immune-histological examination. Thus, a tool such as Opal can be utilised for whole slide scanning and automated analysis of ST infiltrates and tissue phenotype analysis. ST phenotype is thought to influence treatment outcomes in patients, therefore, comprehensive analysis of ST histology will result in more personalised and stratified medicine regimes for patients.
To cite this abstract in AMA style:
Murray-Brown W, Weedon H, Aloia A, Lester S, Proudman S, Smith MD, Walker J, Wechalekar MD. Automated Multiparameter Microscopy and Image Analysis: Next Generation Synovial Tissue Histology [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/automated-multiparameter-microscopy-and-image-analysis-next-generation-synovial-tissue-histology/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/automated-multiparameter-microscopy-and-image-analysis-next-generation-synovial-tissue-histology/