Session Information
Date: Monday, October 22, 2018
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Pediatric localized scleroderma (LS) is typically categorized by the depth and extent of skin lesions into main subtypes: linear scleroderma, including linear trunk/limb and linear head, circumscribed morphea (superficial or deep), generalized plaque morphea, and mixed subtype. It has become increasingly recognized that LS effects more than the skin; greater than 50% of patients are affected by extra-cutaneous manifestations (ECMs), such as musculoskeletal and neurologic involvement. In systemic sclerosis (SSc), a related but typically more serious type of scleroderma, distinctive clinical subsets and specific patterns of organ involvement can be anticipated by autoantibody serological findings, which aid in the treatment and preventive care for these patients. The role of autoantibodies (autoAbs) in LS as a disease risk stratifier or ECM associations has only been partially evaluated with select antibodies. We suggest a wide array of SSc-associated autoAbs to investigate patterns with clinical associations in LS.
Methods:
Plasma from 69 pediatric LS patients and 46 pediatric healthy controls were tested in the for anti-nuclear antibody (ANA) and various SSc-specific and SSc-associated autoAbs including those to extractable nuclear antigens (ENA) in the FIDIS™ Connective Profile, and to those contained in a SSc line immunoassay (LIA) (Euroimmun, Germany) and a MagPix® (Luminex™) addressable laser bead immunoassay (ALBIA), for a total of 29 distinct autoAbs. Normal cutoffs were determined from the healthy pediatric samples (2 SD above the mean) and then applied to the LS samples to determine positivity. Chi-square analysis was used to determine relationships with clinical variables.
Results:
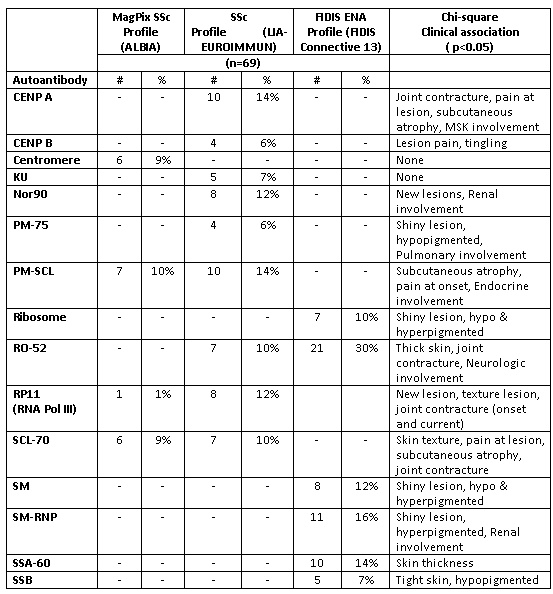
ANA positivity (≥ 1:80 titer) was present in 70% of the LS patients, half of them with a speckled pattern, followed by cytoplasmic (17%), homogenous (12%), nucleolar (10%) and centrosome (9%) pattern. Fifteen of the 28 specific antibodies tested were seen in > 5% of the LS patients and are summarized in Table, along with their significant clinical associations.
Conclusion:
Certain ‘classic’ SSc-associated auto-antibodies, such as Scl-70, centromere (CENP A) and RNA Pol III (RP11) were found in a frequency ranging from 6 – 14%, and these 3 auto-antibodies were associated with deep tissue involvement, signified by joint contractures, nerve entrapment and muscle involvement. Interestingly, none of the 15 autoAb associated with LS subtype designation. Although internal organ involvement is uncommon in LS, when it was present, it did associate with an autoantibody. Therefore, supporting the use of these SSc-associated antibodies as potential classifiers or predictors of disease involvement in LS, though may be different manifestations from SSc. Further analyses of the details of ECMs, and associations of autoAbs with traditional labs (WBC, ESR, CPK), other autoAbs, and cytokines are underway.
To cite this abstract in AMA style:
Porter A, Mirizio E, Fritzler MJ, Brown R, Choi M, Schollaert-Fitch K, Liu C, Torok KS. Autoantibody Testing in Pediatric Localized Scleroderma (LS) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/autoantibody-testing-in-pediatric-localized-scleroderma-ls/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoantibody-testing-in-pediatric-localized-scleroderma-ls/