Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: To describe current bDMARD treatment patterns for ARAD participants with PsA, after combining with linked government PBS data.
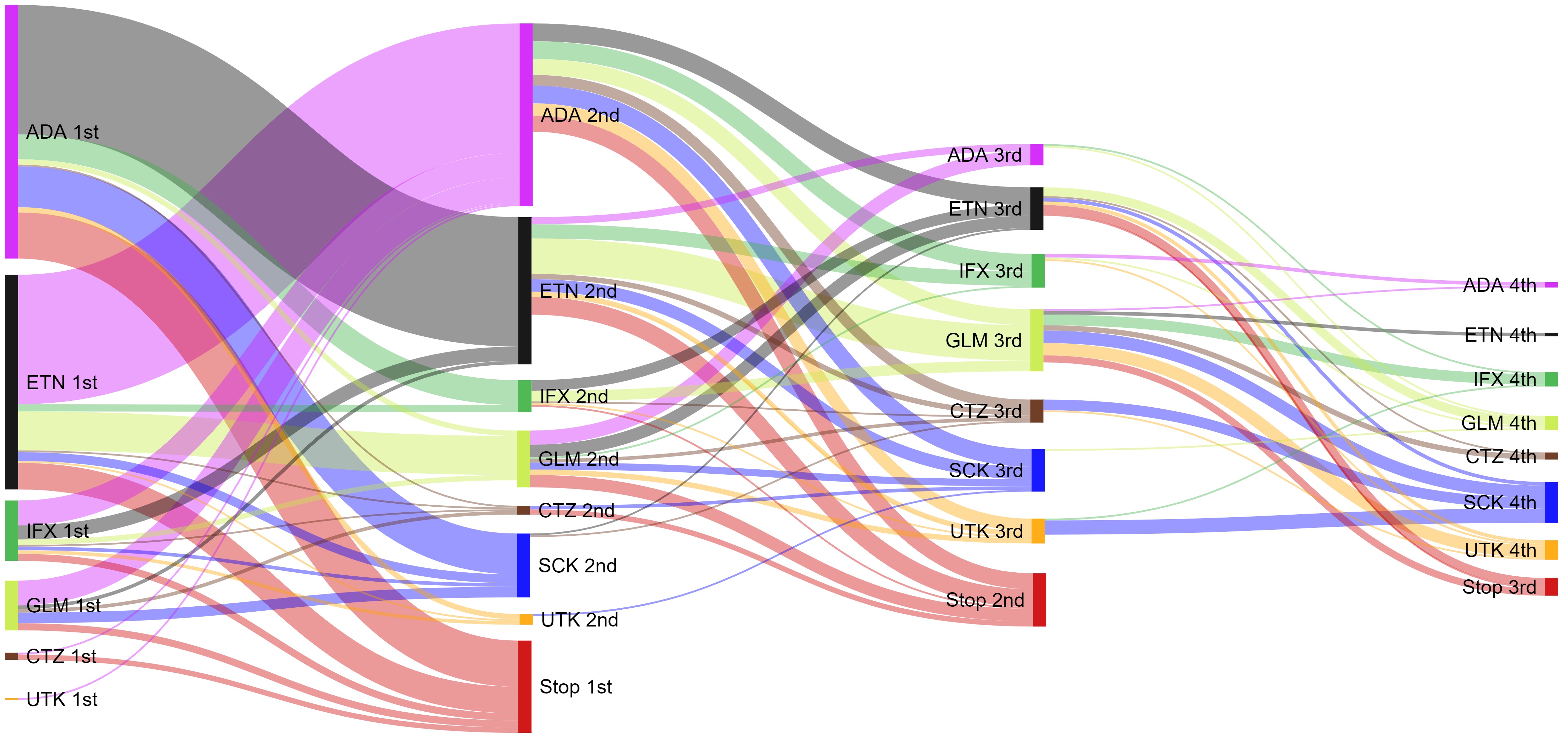
Methods: ARAD, a voluntary longitudinal observational database established in 2001, collects long-term outcome data for people with inflammatory arthritis in Australia. Participants complete semi-annual, then annual questionnaires. Demographic, clinical, drugs, patient-reported outcome (PRO) measures and reasons for stopping bDMARD therapy, such as inefficacy or side-effects, were extracted from Sept. 2001 to June 2020 for all PsA participants. Socioeconomic status (SES) was derived from the participants address and assigned an Australian Bureau of Statistics (ABS) Socio-Economic Indexes for Areas (SEIFA) score of the Index of Relative Socio-economic Advantage and Disadvantage (IRSAD). PBS is the Australian Government program that subsidises medicines, ARAD participants were linked to PBS data from 2011 to 2018. Logistic regression was used to evaluate baseline characteristics of participants who switched compared to those who did not. Switching patterns determined for each bDMARD and time on first, second and third-line bDMARDs were analysed and reasons for switching in the ARAD data were assessed.
Results: 673 PsA participants were included, 5.4% were excluded as ARAD did not match PBS and 31.3% extended medication records beyond the last ARAD questionnaire. First-line bDMARDs were adalimumab (n=283), etanercept (n=178), golimumab (n=44), infliximab (n=32), secukinumab (n=9), certolizumab (n=6) and ustekinumab (n=4). At 1 year 26.9% starting first-line bDMARD therapy switched to another bDMARD, 37.0% switched from second-line therapies and 31.3% switched from third-line therapies. For First-line bDMARDs, females (OR 1.78; 95%CI: 1.18-2.66), higher SES (lowest vs highest OR 2.47; 95%CI: 1.25-4.87), opioid users (OR 1.64; 95%CI: 1.02-2.63) and worse PRO measures (i.e. EuroQol (EQ-5D) OR 0.28; 95%CI: 0.11-0.69) were more likely to switch. Inefficacy (first-line 50.5%, second-line 65.4%, third-line 62.0%) or side effects (first-line 19.0%, second-line 12.0%, third-line 14.0%) were the most commonly cited reasons for stopping therapy irrespective of line of treatment.
Conclusion: Patient self-report medication data in ARAD has a high degree of accuracy when compared with the PBS government database. The treatment pathways for bDMARD use by PsA patients in Australia is complex with 30% switching to another bDMARD therapy after 1 year. Common reasons for switching or stopping were inefficacy and side effects.
To cite this abstract in AMA style:
Fletcher A, March L, Lassere M, Hill C, Barrett C, Carroll G, Sinnathurai P, Buchbinder R. Australian Psoriatic Arthritis (PsA) Biologic Disease-modifying Antirheumatic Drug (bDMARD) Treatment Pathways Using Sankey Diagrams and Predictive Analysis: A Combined Australian Rheumatology Association Database (ARAD) and Pharmaceutical Benefits Scheme (PBS) Analysis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/australian-psoriatic-arthritis-psa-biologic-disease-modifying-antirheumatic-drug-bdmard-treatment-pathways-using-sankey-diagrams-and-predictive-analysis-a-combined-australian-rheumatology-associa/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/australian-psoriatic-arthritis-psa-biologic-disease-modifying-antirheumatic-drug-bdmard-treatment-pathways-using-sankey-diagrams-and-predictive-analysis-a-combined-australian-rheumatology-associa/