Session Information
Date: Sunday, November 8, 2015
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Patients (pts) with psoriatic arthritis (PsA) report poorer health-related quality of life (HRQoL) compared to the general population. As with psoriasis (PSO), it is expected that HRQoL may be worse in PsA pts with skin manifestations affecting visible body areas. Previous reports of RAPID-PsA (NCT01087788) demonstrated improvements in clinical outcomes and HRQoL in PsA pts treated with certolizumab pegol (CZP) over 96 weeks (wks).1 Here we report improvements in skin outcomes by body area affected and investigate associations with HRQoL.
Methods:
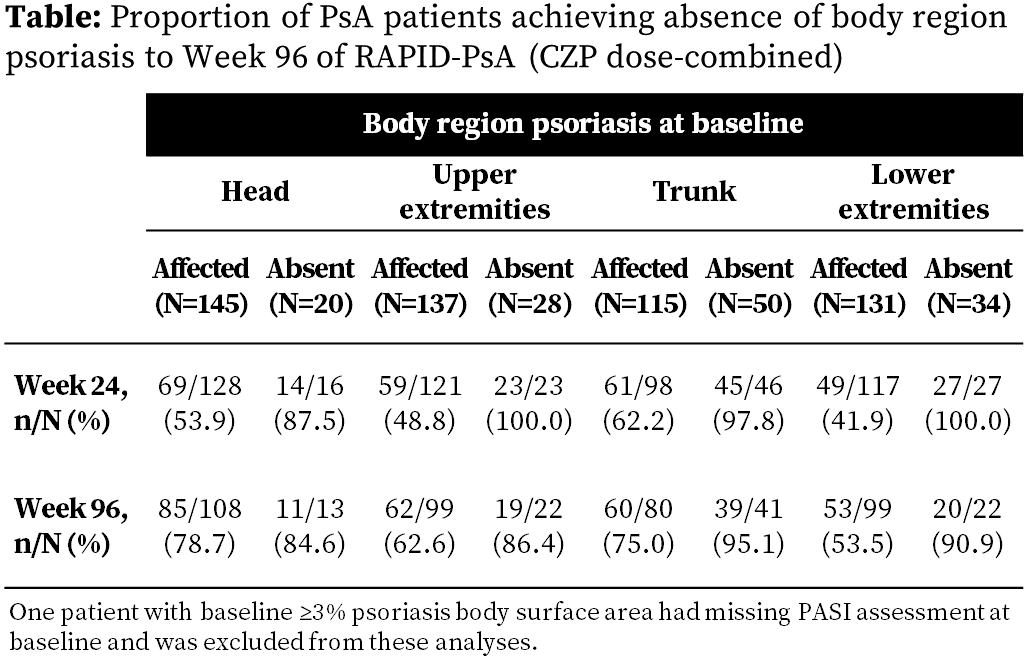
RAPID-PsA was double-blind and placebo (PBO)-controlled to Wk24, dose-blind to Wk48 and open-label to Wk216. Pts had active PsA and failed ≥1 DMARD. Pts were randomized at baseline (BL) to PBO or CZP. Primary clinical endpoint (Wk12 ACR20 response) was reported previously.2 Skin outcomes are presented for Wk0 CZP-randomized pts with ≥3% PSO body surface area (BSA) at BL. PASI scores are presented by body region. Spearman correlations are calculated to assess BL associations between Dermatology Life Quality Index (DLQI) and PASI in all pts with ≥3% PSO BSA at BL. Absence of PSO was defined as PASI=0 by body region and summarized separately by PsA subgroups with/without respective body region PSO at BL. Observed data are shown.
Results:
Of 409 pts randomized, 273 received CZP from Wk0 (166 [60.8%] had ≥3% BSA at BL; mean BL PASI 12.0). RAPID-PsA primary endpoint was met (ACR20 [NRI]: 58.0%, 51.9% vs 24.3%; for CZP 200 mg Q2W, 400 mg Q4W vs PBO). HRQoL improvements to Wk24, assessed by HAQ-DI, were maintained to Wk96 (mean change from BL HAQ-DI [SD]: -0.5 [0.6] at Wk24; -0.6 [0.6] at Wk96).
Improvements in mean PASI body region scores were observed to Wk96 of CZP treatment (mean PASI body region scores at BL vs Wk96 were: Head: 12.0 vs 1.7; Upper extremities: 12.2 vs 1.9; Trunk: 10.8 vs 1.4; Lower extremities: 12.9 vs 2.4).
A moderate, positive correlation was observed at BL between DLQI and total PASI score (r=0.50) and in the PASI body regions of upper extremities, trunk and lower extremities (range, r=0.43–0.50), and a weak, positive correlation was shown between DLQI and PASI head (r=0.28). Correlations between changes in PASI and DLQI to Wk24 were similar to BL correlations.
Over 40% of pts with body region PSO at BL achieved absence of PSO at Wk24; by Wk96, this increased in all regions and was as high as 79% (Table). Overall, only 9 pts without body region PSO at BL developed PSO in that region.
Conclusion:
Improvements in all PASI body region scores were observed in PsA pts following 96 wks of CZP treatment. DLQI had moderate positive correlations with all PASI body regions apart from the head (weakly correlated). At Wk96, absence of body region PSO was attained in at least half of pts with PSO of the respective body region at BL.
References:
- Mease P. Ann Rheum Dis 2014;73(S2):90
- Mease P. Ann Rheum Dis 2014;73(1):48–55
To cite this abstract in AMA style:
Gottlieb AB, Hoepken B, Peterson L, Taylor PC. Associations Between Skin Outcomes By Body Area Affected and Health-Related Quality of Life in Patients with Psoriatic Arthritis Treated with Certolizumab Pegol [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/associations-between-skin-outcomes-by-body-area-affected-and-health-related-quality-of-life-in-patients-with-psoriatic-arthritis-treated-with-certolizumab-pegol/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-between-skin-outcomes-by-body-area-affected-and-health-related-quality-of-life-in-patients-with-psoriatic-arthritis-treated-with-certolizumab-pegol/