Session Information
Session Type: Abstract Session
Session Time: 9:45AM-10:00AM
Background/Purpose: Several antiphospholipid antibody (aPL) profiles are associated with a higher risk for the clinical manifestations of the antiphospholipid syndrome (APS). These include “triple positivity” (lupus anticoagulant [LA], anticardiolipin antibodies [aCL], and anti-b2 glycoprotein I antibodies [ab2GPI]), and LA positivity itself. Further risk is associated with higher levels of aCL and ab2GPI, and with aPL persistence. LA test can detect antibodies to b2GPI and/or prothrombin of any isotype; ab2GPI immunoassays detect isotype-specific antibodies to human b2GPI. Although the putative antigen in aCL tests is cardiolipin, these tests primarily detect isotype-specific antibodies to bovine b2GPI (present in the blocking buffer/sample diluent). Given that the three aPL tests do not detect discrete antibody populations, but rather partially overlapping sets of antibodies, the primary goal of this study was to further characterize the associations among aPL tests using validated APS ACTION Core Laboratory data.
Methods: The APS ACTION Registry was created to study the natural course of persistently aPL-positive patients with or without autoimmune disorders over at least 10 years. The inclusion criteria are positive aPL according to Updated Sapporo Classification Criteria tested within one year prior to enrollment. Patients are followed every 12±3 months with clinical data and blood collection for APS ACTION Core Laboratory aPL confirmation. We analyzed baseline and prospective Core Laboratory aPL data for associations. Spearman’s rank correlation with Bonferroni adjusted significance level for multiple comparisons was used to assess correlation between all available aPL ELISA test results (Inova Diagnostics). Univariate logistic regression was used to assess laboratory predictors of positive LA test.
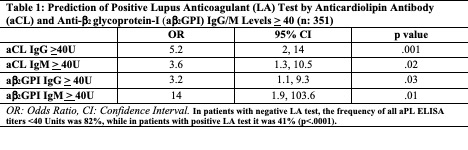
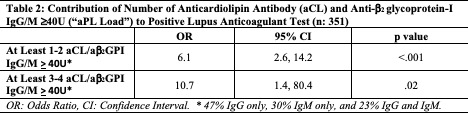
Results: As of 1/2021, 854 patients were included; 567 patients had Core Laboratory aPL profiles at baseline and follow up. Based on four (aCL/ab2GPI IgG/M) same sample tests (n: 1008) at baseline and follow up, a strong correlation was identified between aCL IgG and ab2GPI IgG, and aCL IgM and ab2GPI IgM (r=0.75, p< .001 for both). There was no correlation between IgG and IgM isotypes. Based on five (aCL/ab2GPI IgG/M and LA) same sample tests at baseline, patients with: a) aCL/ab2GPI IgG/IgM >40U had a higher chance of a positive LA test, compared to those with lower titers (Table 1); b) one or two positive tests > 40U among aCL/ab2GPI IgG/IgM had 6.1 times higher odds of a positive LA test; and c) three or four positive tests > 40U among aCL/ab2GPI IgG/IgM had 10.7 times higher odds of a positive LA test (Table 2). The frequencies of aCL IgG, ab2GPI IgG, aCL IgM, and ab2GPI IgM levels >40U in LA-positive and LA-negative patients are shown in Figure 1.
Conclusion: Using a large scale international aPL/APS database, we confirmed a strong association between aCL IgG and ab2GPI IgG and, similarly, aCL IgM and ab2GPI IgM, that is likely explained by the b2GPI dependence of aCL tests. Both aPL ELISA levels > 40U and a higher number of aPL ELISA tests > 40U were predictive of a positive LA test, introducing the concept of “aPL load”, that may provide a mechanistic explanation of a positive LA test.
To cite this abstract in AMA style:
Gkrouzman E, Andrade D, Tektonidou M, Pengo V, Ugarte A, Belmont H, Chighizola C, Fortin P, Atsumi T, Efthymiou, M, de Jesus G, Branch D, Andreoli L, Petri M, Rodriguez-Almaraz E, Cervera R, Knight J, Gonzalez E, Bison E, Mackie I, Cohen H, Bertolaccini M, Erkan D, Roubey R, APS ACTION o. Associations Among Antiphospholipid Antibody Types, Isotypes, and Titers: Results from the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/associations-among-antiphospholipid-antibody-types-isotypes-and-titers-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinical-dat/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-among-antiphospholipid-antibody-types-isotypes-and-titers-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinical-dat/