Session Information
Session Type: Abstract Submissions (ACR)
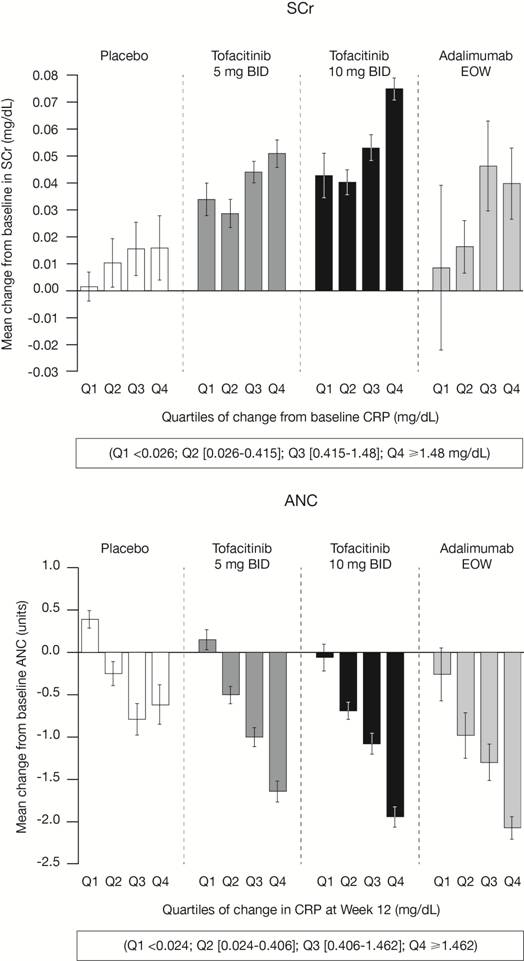
Background/Purpose: Tofacitinib is a novel, oral Janus kinase (JAK) inhibitor for the treatment of rheumatoid arthritis (RA). Changes in laboratory parameters observed during tofacitinib treatment included mean increases in low (LDL) and high (HDL) density lipoproteins, serum creatinine (SCr) and mean decreases in neutrophils (ANC). Potential explanatory mechanisms were investigated.
Methods: Baseline and Week 12 data from five Phase 3 (P3) traditional or biologic disease-modifying anti-rheumatic drug (DMARD)-inadequate responder (IR) trials were pooled for each treatment arm: placebo, tofacitinib 5 mg and 10 mg twice daily, and adalimumab 40 mg every other week. The defined laboratory parameters were explored with C-reactive protein (CRP) as a marker of inflammation. Mean changes at Week 12 were compared with quartile levels of CRP at baseline and quartiles of change in CRP at Week 12.
Results: Across the defined laboratory parameters, the smallest mean changes from baseline were observed in the quartile of patients with the smallest reductions in CRP (Figure, 1st quartile) and the greatest changes were observed with the greatest reductions in CRP (Figure, 4th quartile). A similar pattern of association was evident with baseline CRP, where the greatest mean changes in laboratory parameters occurred in patients with highest levels of baseline CRP. Numerical differences in the magnitude of changes across the treatment arms were observed, but no statistical comparisons were performed.
Conclusion: A consistent pattern of association between mean changes in each of the laboratory parameters and CRP was observed. The precise mechanism behind these laboratory changes is unknown. Based upon these analyses, the lowering of inflammation, as measured by CRP, may partly explain some of the observed mean changes in laboratory parameters during clinical studies.
Disclosure:
V. Strand,
Abbott Immunology Pharmaceuticals, Amgen Inc, AstraZeneca, Biogen Idec, Canfite Pharma, Centocor Inc, Cypress Biosciences Inc, Euro-Diagnostica Inc, Fibrogen, Forest Laboratories, Genentech, Human Genome Sciences Inc, Incyte, Novartis Pharmaceuticals Corp,
5;
J. D. Isaacs,
Pfizer Inc,
2,
Pfizer Inc,
5,
Pfizer Inc,
8;
S. Menon,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
J. Beal,
Pfizer Inc,
1,
Pfizer Inc,
3;
C. I. Nduaka,
Pfizer Inc,
1,
Pfizer Inc,
3;
S. Krishnaswami,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
R. Riese,
Pfizer Inc,
1,
Pfizer Inc,
3;
M. G. Boy,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-mean-changes-in-laboratory-safety-parameters-with-c-reactive-protein-at-baseline-and-week-12-in-rheumatoid-arthritis-patients-treated-with-tofacitinib/