Session Information
Date: Sunday, November 8, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Oral glucocorticoids are commonly
used to treat rheumatoid arthritis (RA). However, their use may be associated
with potential adverse events. Therefore, the objective was to evaluate the
association between exposure to oral glucocorticoids and incident adverse
events, using three different measures of oral glucocorticoid exposure.
Methods: This retrospective cohort study
utilized two large administrative claims databases. Patients with RA aged 18
years and older with at least one prescription claim for an oral glucocorticoid
between 1/1/2010-12/31/2013 (index date) were identified. Patients were
required to be continuously enrolled for 12 months prior to index date with no
claims for oral glucocorticoids, to be continuously enrolled for 1 month after
index date and have no diagnoses for other autoimmune conditions. Oral
glucocorticoid use was measured in monthly panel periods after the index date.
The following measures were captured: cumulative dose in prednisone-equivalent
milligrams, months on therapy, and months since last use. Presence and time
from index date to any potential adverse event assessed (osteoporosis,
fracture, aseptic necrosis of bone, hospitalization for pneumonia or infection,
hospitalization for myocardial infarction or stroke, type 2 diabetes,
ulcer/gastrointestinal bleed) were measured. Cox proportional hazards models
with time-fixed and time-varying covariates were fit to the data.
Results: There were 36,502 RA patients
identified; average age 58.2 years and 78.3% female. Patients were followed for
a total of 60,663 years. During follow-up, 17,512 patients (48.0%) had at least
3 months with oral glucocorticoid exposure. Mean cumulative dose/month was 59
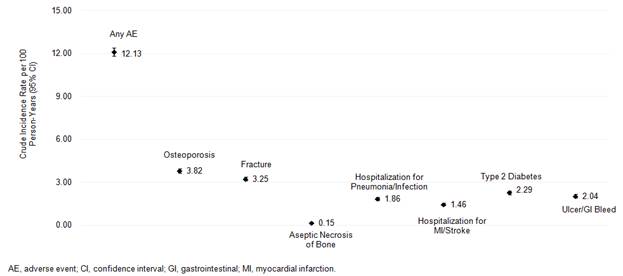
mg. 7,358 (20.2%) patients had evidence of a potential adverse event. Unadjusted
incidence rates are presented in Figure 1. Results from time-varying models are
presented in Table 1. Higher cumulative dose and more months of treatment were
associated with a significantly increased risk of adverse event, while an
increase in the time since last glucocorticoid use was associated with a
significantly decreased risk of adverse event. These trends were noted in
individual models and in models including all three exposure measures.
Conclusion: Among patients with RA, greater
exposure to oral glucocorticoids, operationalized as higher cumulative dose and
more months of treatment, was associated with increased risk of experiencing an
adverse event. Greater time since last exposure was associated with a decreased
risk. It is important for clinicians to consider steroid-sparing treatments for
RA patients.
Figure 1.
Table 1.
|
|
Hazard Ratio (95% Confidence Interval) |
p-value |
|
Model 1a: Unadjusted |
|
|
|
Additional 100 mg in cumulative dose |
1.0067 (1.0054-1.0080) |
<0.0001 |
|
|
|
|
|
Model 1b: Adjusted* |
|
|
|
Additional 100 mg in cumulative dose |
1.0055 (1.0042-1.0068) |
<0.0001 |
|
|
|
|
|
Model 2a: Unadjusted |
|
|
|
Additional month with use |
1.0311 (1.0253-1.0369) |
<0.0001 |
|
|
|
|
|
Model 2b: Adjusted* |
|
|
|
Additional month with use |
1.0233 (1.0176-1.0292) |
<0.0001 |
|
|
|
|
|
Model 3a: Unadjusted |
|
|
|
Additional month since last use |
0.9837 (0.9798-0.9877) |
<0.0001 |
|
|
|
|
|
Model 3b: Adjusted* |
|
|
|
Additional month since last use |
0.9847 (0.9807-0.9887) |
<0.0001 |
|
|
|
|
|
Model 4a: Unadjusted |
|
|
|
Additional 100 mg in cumulative dose |
1.0030 (1.0010-1.0049) |
0.0034 |
|
Additional month with use |
1.0184 (1.0104-1.0264) |
<0.0001 |
|
Additional month since last use |
0.9914 (0.9869-0.9959) |
0.0002 |
|
|
|
|
|
Model 4b: Adjusted* |
|
|
|
Additional 100 mg in cumulative dose |
1.0029 (1.0011-1.0048) |
0.0021 |
|
Additional month with use |
1.0099 (1.0021-1.0178) |
0.0128 |
|
Additional month since last use |
0.9901 (0.9856-0.9946) |
<0.0001 |
|
*Adjusted for time-varying biologic and non-biologic DMARD use and time-fixed age, gender, region, health plan type, urbanicity, payer, Charlson Comorbidity Index, number of unique 3-digit diagnosis codes, number of unique National Drug Codes, asthma diagnosis, cancer diagnosis, hypothyroidism diagnosis, ischemic heart disease diagnosis, and Paget’s disease/osteomalacia diagnosis. |
||
To cite this abstract in AMA style:
Best J, Farr A, Lenhart G, Sarsour K, Stott-Miller M, Hwang YG. Association Between Three Measures of Oral Glucocorticoid Exposure and Potential Adverse Events Among Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/association-between-three-measures-of-oral-glucocorticoid-exposure-and-potential-adverse-events-among-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-three-measures-of-oral-glucocorticoid-exposure-and-potential-adverse-events-among-patients-with-rheumatoid-arthritis/