Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Plasminogen activator inhibitor 1 (PAI-1), a SERPIN-type inhibitor, is best known for regulating fibrinolysis, where it inhibits the activity of tissue and urokinase plasminogen activators. High circulating PAI-1 is a risk factor for thrombosis in the general population. Furthermore, recent studies have suggested that PAI-1 plays a pathogenic role in various rheumatic diseases, for example, scleroderma-associated fibrosis. Whether PAI-1 is a key player in antiphospholipid syndrome (APS) pathogenesis remains an unsettled question.
Methods: To determine whether PAI-1 is expressed by vessel-resident cells in APS, single-cell RNA sequencing (scRNA-seq) was performed on the dermal tissue of 3 APS patients with livedo racemosa and 4 healthy age-matched controls. Plasma, along with clinical and laboratory parameters, were collected from 167 APS patients and 37 antiphospholipid antibody (aPL)-positive carriers. Total PAI-1 and active PAI-1 were evaluated in plasma by a Luminex-based assay with active PAI-1 determined through a unique urokinase plasminogen activator-based assay. Plasma biomarkers, including P-selectin, E-selectin, ICAM-1, and VCAM-1 were tested by traditional ELISA. Healthy microvascular endothelial cells (MVECs) were cultured with APS patient-derived IgG, and PAI-1 expression was measured by quantitative PCR and ELISA.
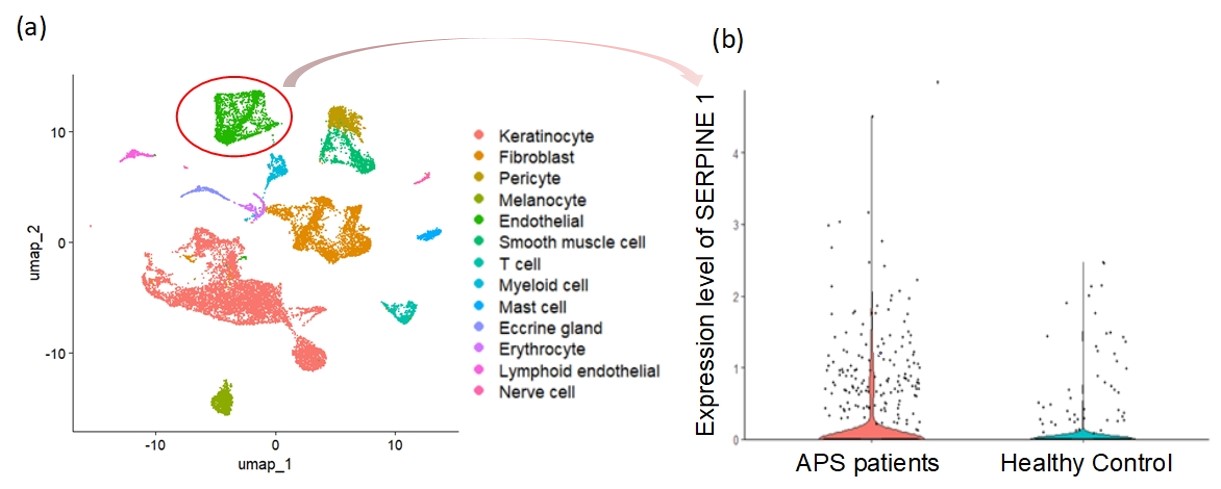
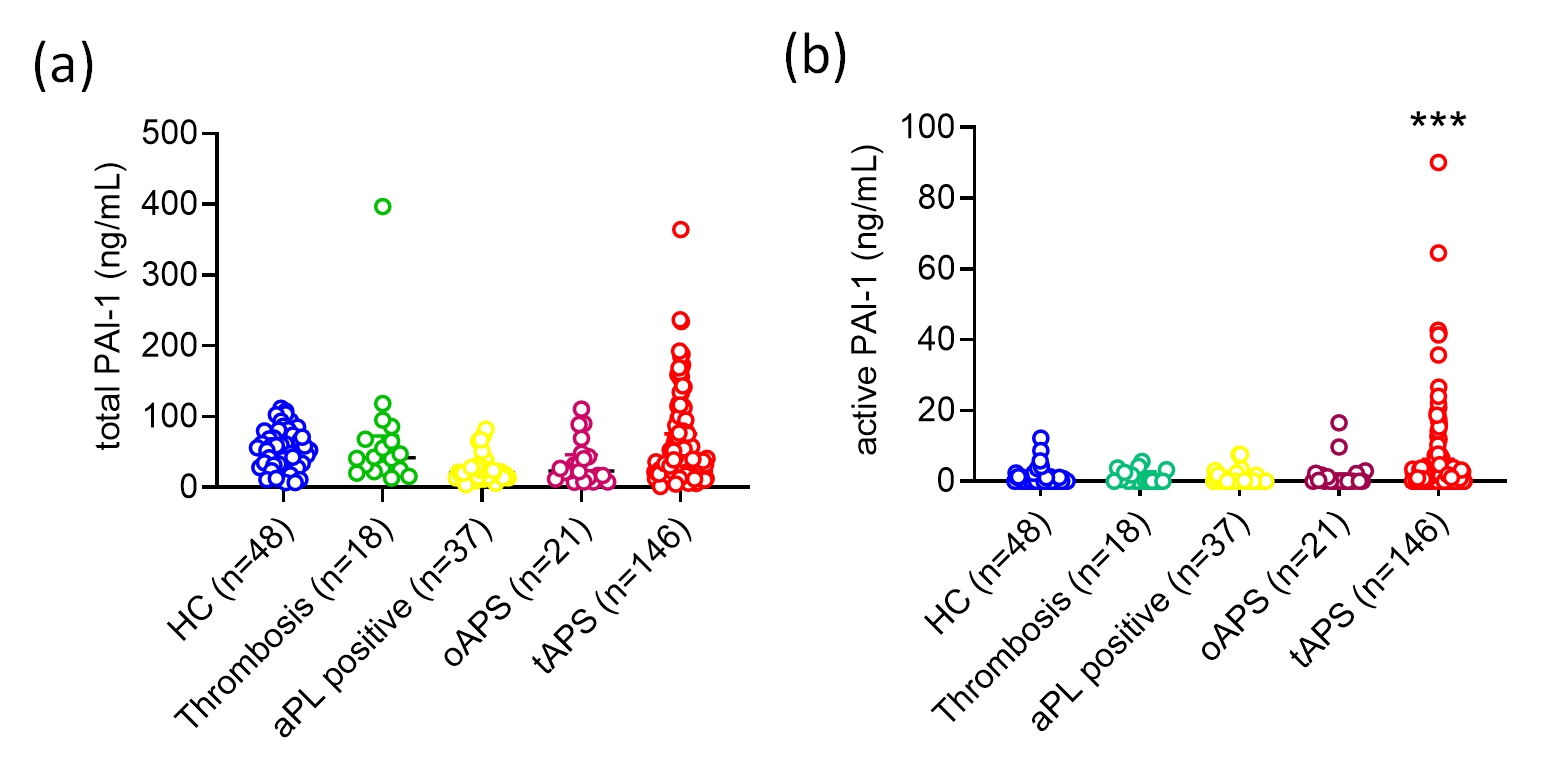
Results: Analysis of the scRNAseq data revealed that skin cells from APS patients and healthy controls could be clustered into 13 unique cell population groups (Fig. 1a), including endothelial cells. As compared with control endothelial cells, the gene for PAI-1 (SERPINE1) was upregulated by 2.4-fold (adjusted P< 0.001) in APS endothelial cells (Fig. 1b). Active PAI-1, but not total PAI-1, was significantly elevated in the plasma of patients with thrombotic APS (tAPS), but not patients with obstetric APS, aPL carriers, non-APS thrombotic controls, or healthy controls (Fig. 2a-b). In patients with tAPS, active PAI-1 was positively associated with neutrophil count (rs=0.193, P=0.039), CRP (rs=0.275, P=0.006), ESR (rs=0.210, P=0.040), and complement C3 (rs=0.381, P < 0.001). Furthermore, patients with tAPS who had a greater aPL burden (aGAPSS ≥7) had more active PAI-1 in their plasma than those with lower aGAPSS [0.87 (0, 7.34) ng/mL vs. 0 (0, 2.33) ng/mL, P=0.022]. Compared with healthy controls, plasma P-selectin, E-selectin, ICAM-1, and VCAM-1 were all elevated in patients with tAPS. Of these biomarkers, only E-selectin was significantly associated with active PAI-1 (rs=0.336, P< 0.001). Culture of MVECs with APS patient-derived IgG led to upregulation of PAI-1 mRNA (fold-change 1.93 ± 0.09, P=0.006) and more secretion of PAI-1 into culture supernatants (1.62 ± 0.14 ng/mL vs. 1.08 ± 0.13 ng/mL, P=0.015).
Conclusion: In vivo and in vitro evidence suggests that endothelial cells are likely an important source of PAI-1 in APS. In plasma, PAI-1’s active form was preferentially elevated in patients with tAPS, compared with the other characterized groups. Experiments are now underway to determine the extent to which PAI-1 may be a viable therapeutic target in animal models of the disease.
To cite this abstract in AMA style:
Gan Y, Liang W, Warnock M, Yalavarthi S, Sarosh C, Tambralli A, Madison J, Zuo Y, Lawrence D, Knight J. Association Between the Active Form of Plasminogen Activator Inhibitor 1 (PAI-1) and Endothelial Cell Activation in Antiphospholipid Syndrome [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/association-between-the-active-form-of-plasminogen-activator-inhibitor-1-pai-1-and-endothelial-cell-activation-in-antiphospholipid-syndrome/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-the-active-form-of-plasminogen-activator-inhibitor-1-pai-1-and-endothelial-cell-activation-in-antiphospholipid-syndrome/