Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The primary efficacy measure in urate lowering therapy (ULT) trials is usually serum urate (SU). However, it is unknown whether the strength of the association between SU and clinically relevant outcomes is sufficient for SU to be considered a surrogate. The aim of this study was to examine the strength of a possible association between SU and flares, indirectly providing support for SU as an important biomarker in clinical practice.
Methods: A systematic literature review was undertaken to identify all relevant studies. First, randomized controlled trials (RCTs) comparing any ULT in people with gout with any control or placebo, ≥3months duration were included. The maximum RCT duration of 12 months and studies where the proportion of individuals with SU<6mg/dL and the proportion having a flare were assessed in close temporal proximity may obscure any relationship. Therefore, open label extension (OLE) and longitudinal observational studies were included in secondary analyses. Standardized data elements were extracted by two independent reviewers. Association between the variables were analyzed by simple correlation between gout flare rate either as a proportion or converted into events over patient-years (dependent variable[s]), scattered against the proportion achieving SU <6mg/dL and the duration of the trial. The CORR procedure (SAS) provided a nonparametric measure of association between the variables; we used Spearman’s rank-order correlation to test the potential association between variables.
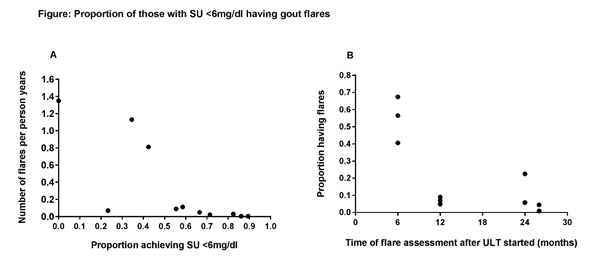
Results: Of the 2,775 records, 9 RCTs were identified. 3 OLE studies and 11 observational studies were identified. Using data from the 9 RCTs, of which the maximum trial duration was 12 months, meta-regression analysis did not reveal a statistically significant association between the proportion of individuals with SU<6mg/dL and the observed flare rate (P=0.82). The ratio of the time in months at which the proportion of individuals having a flare was reported/time in months at which the proportion of individuals with SU<6mg/dL was reported was calculated and those studies where the ratio was <2 were excluded. Using the remaining 5 studies (incl. 11 data points) there was a clear association between proportion of individuals achieving SU<6mg/dL and the observed gout flares (over patient years; Fig A; Spearman rho= -0.888, P=0.0003). Duration of ULT was also strongly inversely associated with the proportion of patients experiencing a flare (Fig B; Spearman rho= -0.878, P= 0.0004).
Conclusion: From observational data, SU <6mg/dL is strongly associated with reduced gout flares but the duration of the RCTs needed to reveal possible causation, with clinically relevant benefit takes longer than the usual RCT duration. These associations provide some support that SU may be a suitable biomarker for gout clinical trials.
To cite this abstract in AMA style:
Stamp LK, Morillon M, Taylor W, Dalbeth N, Lassere M, Singh JA, Christensen R. Association between Serum Urate As a Surrogate Endpoint and Flares in People with Gout: An Ecological Study Based on a Systematic Review of Trials and Open Label Extensions [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/association-between-serum-urate-as-a-surrogate-endpoint-and-flares-in-people-with-gout-an-ecological-study-based-on-a-systematic-review-of-trials-and-open-label-extensions/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-serum-urate-as-a-surrogate-endpoint-and-flares-in-people-with-gout-an-ecological-study-based-on-a-systematic-review-of-trials-and-open-label-extensions/