Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders I: Clinical Trials
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: The SEMAPHORE trial1 was a randomized controlled prospective study to assess the safety and efficacy (success defined by PMR-AS≤10 and GC≤5mg or GC decrease ≥10mg/day) of intra venous (i.v.) tocilizumab (TCZ) in glucocorticoids (GC)-dependent polymyalgia rheumatica (PMR). Tocilizumab was shown to reduce GC while improving clinical and biological inflammatory parameters at 24 weeks. After the 24-week study, patients entered a double-blind extension.
Objectives
In this study, we aimed to assess after the 24th week the relapse rate and markers of patients who received a 6-month TCZ treatment and achieved remission.
Methods:
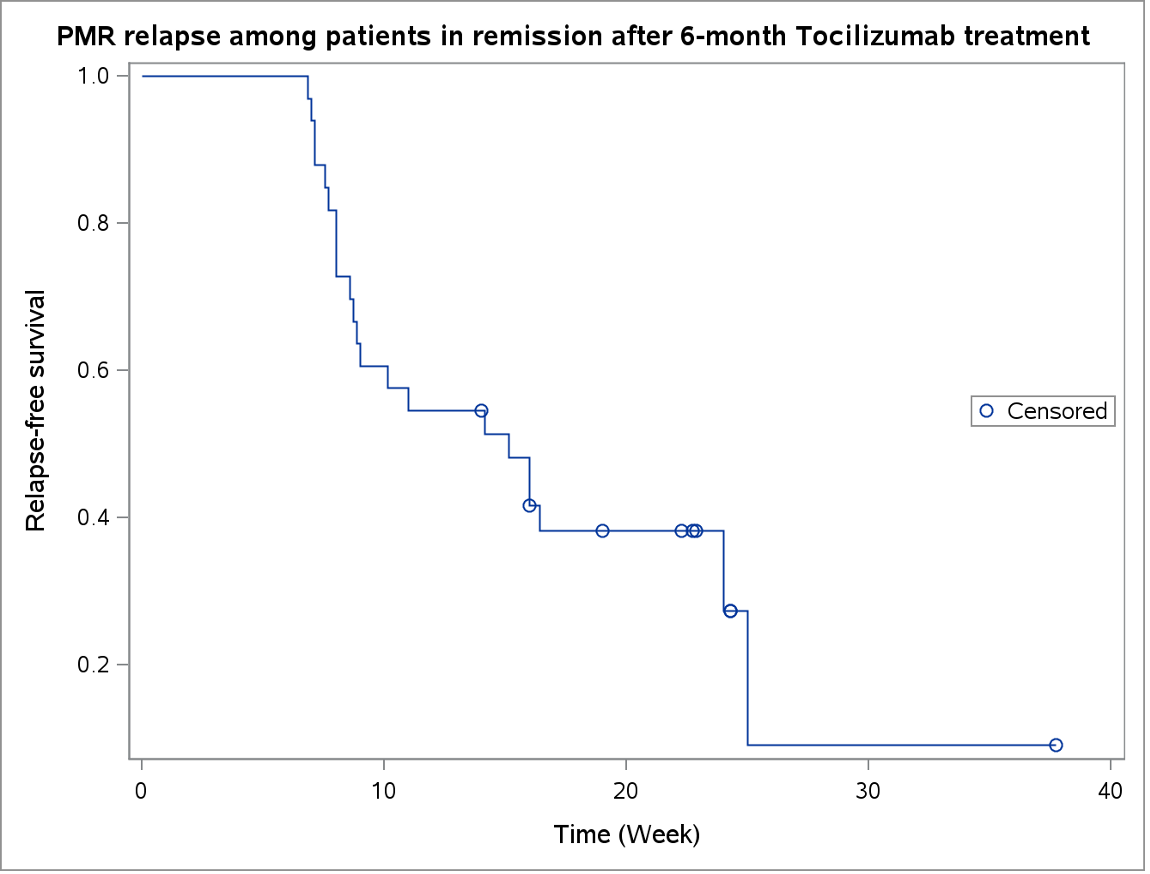
Among 101 patients randomly 1:1 allocated to receive i.v. 8mg/kg TCZ or placebo for 24 weeks in the SEMAPHORE trial, 49 received TCZ and 33 of them succeed . All 33 patients but one stopped TCZ at week 24 and they visited at week 32, with optional follow-up visits every 8 weeks until week 48. The relapse was defined by the failure of the primary composite outcome (PMR-activity score ≤10 while glucocorticoids decreased ≤5mg/day or glucocorticoids decrease was ≥10mg/day compared to inclusion) during follow-up or the need for ≥1 TCZ infusion. Results are presented by proportion of patients achieving success at each visit and the outcome over time using a Kaplan Meier curve.
Results: Among the 33 TCZ group patients in remission at week 24, 7 stopped follow-up before the 48th week, 5 of the other 26 subjects (19.2%) sustained remission in the 6 following months, 21 (80.8%) relapsed. Median time to relapse was 15 weeks (interquartile range: 8-25).
15 patients were considered in relapse because of PMR-AS≥10, 4 for isolated CRP elevation, 2 after the clinician’s decision.
Among the 16 patients treated with TCZ but not in remission at 24th week, 4 of them (25%) achieved the primary outcome after continuing TCZ for 24 other weeks.
Conclusion
Among patients with GC-dependent PMR, in remission after a 6-month TCZ treatment, only a quarter remained relapse-free after treatment discontinuation. This study suggests that a 6-month treatment is not enough to withdraw TCZ. Further studies assessing the required duration of anti-IL6-receptor treatment to limit PMR relapses are needed.
Conclusion: Among patients with GC-dependent PMR, in remission after a 6-month TCZ treatment, only a quarter remained relapse-free after treatment discontinuation. This study suggests that a 6-month treatment is not enough to withdraw TCZ. Further studies assessing the required duration of anti-IL6-receptor treatment to limit PMR relapses are needed.
Footnotes: Remission was defined by PMR-AS<10, and GC≤5mg/j or daily GC dose decreased by ≥10mg. Day 0 was the visit at 24th week in the SEMAPHORE trial
Abbreviations: PMR: Polymyalgia rheumatica; PMR-AS: Polymyalgia rheumatica activity score
To cite this abstract in AMA style:
Chevet B, Souki A, emmanuel n, CARVAJAL ALEGRIA G, Dernis E, Richez C, Truchetet M, WENDLING D, TOUSSIROT E, Perdriger a, Gottenberg J, FELTEN R, Fautrel B, chiche l, Walliser-Lohse A, Hilliquin P, Le Henaff C, Dervieux B, Direz G, Valckenaere I, Cornec D, Guellec D, Marhadou T, SARAUX A, Devauchelle V. Assessment of the Remission Maintenance After Tocilizumab Withdrawal in Polymyalgia Rheumatica Patients Receiving a 6-month Treatment [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/assessment-of-the-remission-maintenance-after-tocilizumab-withdrawal-in-polymyalgia-rheumatica-patients-receiving-a-6-month-treatment/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-the-remission-maintenance-after-tocilizumab-withdrawal-in-polymyalgia-rheumatica-patients-receiving-a-6-month-treatment/