Session Information
Date: Monday, November 13, 2023
Title: (1554–1578) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Damage is the irreversible consequence of disease or its treatment. This study aimed to evaluate the accrual of damage in Takayasu’s arteritis (TAK) using 2 currently-available indices of damage.
Methods: Patients with TAK enrolled in a multicenter, prospective, observational cohort from North America, and, a single-center in Turkey were included. Damage was assessed using the Vasculitis Damage Index (VDI) and the Large-Vessel Vasculitis Index of Damage (LVVID) at baseline and last visit.
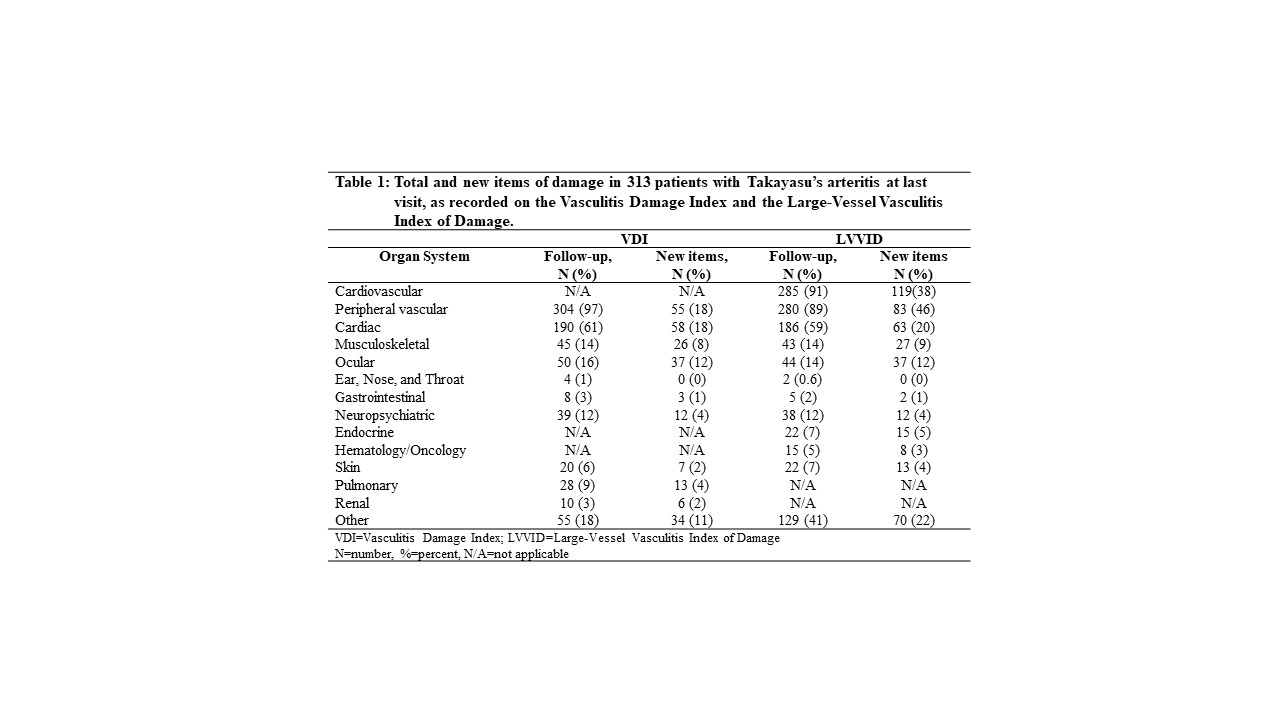
Results: The study included 350 patients with TAK, mean±SD age 40.3 (13.4) years, 91% female, median (25th, 75th percentile) disease duration 69 (1.6, 274) weeks. 39% were enrolled ≤180 days from diagnosis. 313 (89%) had at least 1 follow-up visit, median (25th, 75th) duration 4.5 (2.1, 8.0) years. Damage was present at first visit in 83% on VDI, 89% on LVVID with median VDI (range) 3 (0, 10) and median (range) number of damage items on LVVID 3 (0, 13). Most items of damage at baseline visit were captured in the peripheral vascular (83% VDI, 89% LVVID) and cardiac (42% VDI, 44% LVVID) categories. At last follow-up, damage items were noted in 95% on VDI and 95% on LVVID mainly in the peripheral vascular (97% VDI, 89% LVVID) and cardiac (60% VDI, 59% LVVID) categories (Table 1). New damage was captured in 52% patients on VDI and 53% on LVVID. In patients with new items, median (range) number of new items was 1 (range 1, 13) on VDI and 2 (1, 17) on LVVID (Table 1). The majority of new items of damage were disease-related and were in the peripheral vascular (18% VDI), and cardiac (18% VDI) categories on VDI and cardiovascular category (37% total, 26% peripheral vascular, 20% cardiac) and “Other” (22%) categories on LVVID (Table 1). The most frequent items of damage at last visit in the most frequently affected categories, and, new items of damage at last visit are in Table 2. Damage captured on VDI but not LVVID included major vessel stenosis (83%), pulse loss (98%), elevated diastolic blood pressure (40%), second pulse loss (12%); items of damage captured on LVVID but not VDI included hypertension (40%), arterial thrombosis (41%), renal artery stenosis (28%), damage requiring vascular intervention (8% angioplasty alone, 20% angioplasty with stent), bypass surgery (22%), aortic aneurysms (14%). 12 of 64 items (19%) on VDI and 20 of 87 items (23%) on LVVID were never applicable to any patient with TAK but 13 of the 20 (65%) items not used on LVVID were in the ocular category with manifestations that can occur in giant cell arteritis.
Conclusion: Damage is present in >80% of patients with TAK at first visit. During follow-up, new damage items, mostly disease related, are observed in 50% including new cardiovascular damage in nearly 40%. LVVID captures new damage items in the cardiovascular category more comprehensively than VDI but many damage items were captured in the “Other” category on both measures. Based on these results, LVVID can be modified and streamlined to more efficiently measure damage in TAK and perhaps be focused on disease-related damage. Disease-associated damage accrues in TAK despite treatment and assessment of damage should be included in therapeutic trials.
To cite this abstract in AMA style:
Kermani T, KAYMAZ-TAHRA S, Cuthbertson D, Khalidi N, Koening C, Langford C, McAlear C, Monach P, Moreland L, Pagnoux C, Seo P, Sreih A, Warrington K, Alibaz-Öner f, Direskeneli H, Merkel P. Assessment of the Extent and Accrual of Damage in Takayasu’s Arteritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/assessment-of-the-extent-and-accrual-of-damage-in-takayasus-arteritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-the-extent-and-accrual-of-damage-in-takayasus-arteritis/