Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Pain is commonly cited by patients with PsA as affecting their daily activities and quality of life and may differ by sex1. In addition to neurological pathways, pain signaling involves many cytokines, some of which are tyrosine kinase 2 (TYK2) mediated and involved in PsA pathogenesis. Deucravacitinib is a first-in-class, oral, selective, allosteric inhibitor of TYK2 approved in multiple countries for adults with moderate to severe plaque psoriasis.2,3 Deucravacitinib was efficacious vs placebo (PBO) in a phase 2 trial in patients with active PsA, and certain cytokine levels associated with pain signaling were reduced with deucravacitinib vs PBO, demonstrating the downstream effects of TYK2 inhibition on these pathways.4,5 Here, we characterize the effect of deucravacitinib on pain across different instruments by sex, and alignment across those pain instruments, in patients in the phase 2 PsA trial (NCT03881059).
Methods: Patients with PsA (N=203) were randomized 1:1:1 to PBO, deucravacitinib 6 mg once daily (QD), or deucravacitinib 12 mg QD. Three instruments assessed pain up to week 16: (1) Patient Global Assessment of Pain visual analog scale (Pain VAS), scored from 0-100; (2) Psoriatic Arthritis Impact of Disease (PsAID) pain instrument, scored from 0-10; and (3) 36-Item Short Form Survey (SF-36) Bodily Pain questionnaire, in which patients rated their pain on a 6-item scale from “none” to “very severe.” Mean change from baseline (BL) in pain scale scores by sex, the proportion of patients who reported meaningful improvements in pain, and correlation among pain scales and disease efficacy measures were evaluated.
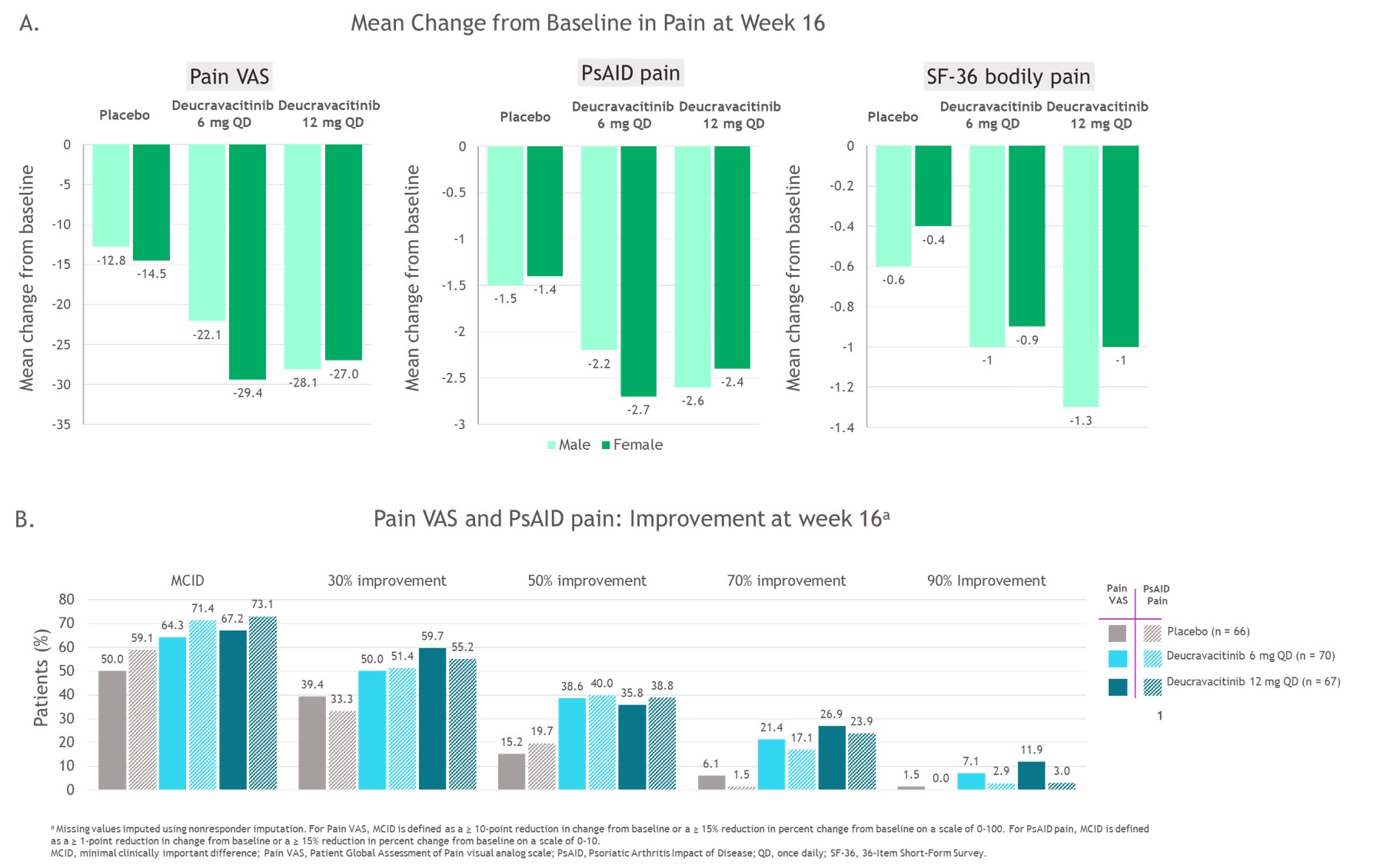
Results: Mean Pain VAS score was 64.1, and mean PsAID pain score was 6.4 at BL; scores were similar across groups. At BL, pain assessments strongly correlated with the Psoriatic Arthritis Disease Activity Score (PASDAS) and Patient Global Assessment of Disease Activity (Table). Mean improvements in pain for all 3 measures and percentages of patients reporting improvements in Pain VAS and PsAID pain scores were greater with deucravacitinib compared with PBO (Figure 1). Similar improvements in pain were observed in both male and female patients. Pain assessments correlated with one another both at BL (Table) and over time through week 16, with some divergent responses to pain questions also observed (Figure 2).
Conclusion: A higher proportion of patients with PsA treated with deucravacitinib reported clinically meaningful improvements in pain compared with PBO. Patient-reported pain assessments had moderate correlation with one another. No consistent differences were observed between male and female patients in reported mean improvements in pain.
References:
1.Coates L, et al. J Rheumatol 2023; 50(4):488–496. 2.Armstrong A, et al. J Am Acad Dermatol 2023;88:29–39. 3.Strober B, et al. J Am Acad Dermatol 2023;88:40–51. 4.Mease PJ, et al. Ann Rheum Dis 2022;81:815–822. 5. FitzGerald O, et al. 2021 ACR Convergence, Abstract 0490; 2021.
To cite this abstract in AMA style:
Mease P, Eder L, Ogdie A, Deodhar A, Banerjee S, Nowak M, Choi J, Lehman T, Strand V. Assessment of Pain Outcomes in a Phase 2 Trial of a Selective, Allosteric Tyrosine Kinase 2 Inhibitor, Deucravacitinib, in Patients with Active PsA [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/assessment-of-pain-outcomes-in-a-phase-2-trial-of-a-selective-allosteric-tyrosine-kinase-2-inhibitor-deucravacitinib-in-patients-with-active-psa/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-pain-outcomes-in-a-phase-2-trial-of-a-selective-allosteric-tyrosine-kinase-2-inhibitor-deucravacitinib-in-patients-with-active-psa/