Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Zasocitinib (TAK-279) is an oral, highly selective, allosteric tyrosine kinase 2 (TYK2) inhibitor that binds with high specificity to the Janus homology 2 domain of TKY2 but not Janus kinase family members, blocking downstream proinflammatory signaling. In a phase 2b trial in patients with active psoriatic arthritis (PsA), a significantly greater proportion of patients achieved an ACR20 response at week 12 after receiving once-daily (QD) oral zasocitinib 15mg or 30mg than placebo (PBO, both p=0.002);1 zasocitinib had an acceptable safety profile. Here, we report an assessment of changes in key laboratory parameters from this study.
Methods: This was a phase 2b, randomized, multicenter, double-blind, placebo-controlled, multiple-dose study (NCT05153148). Patients (18–70 years) with active PsA were randomized 1:1:1:1 to receive either PBO or zasocitinib 5mg, 15mg, 30mg orally QD for 12 weeks.1 Laboratory parameters were assessed at various time points during the study and included hematology, serum chemistry and lipids. The severity of adverse events relating to laboratory parameter changes was classified using Common Terminology Criteria for Adverse Events (CTCAE; version 5.0) and was assessed in the safety analysis set (those who received ≥1 dose of study drug). Shifts from baseline to lowest or highest post-baseline values in these laboratory parameters were assessed.
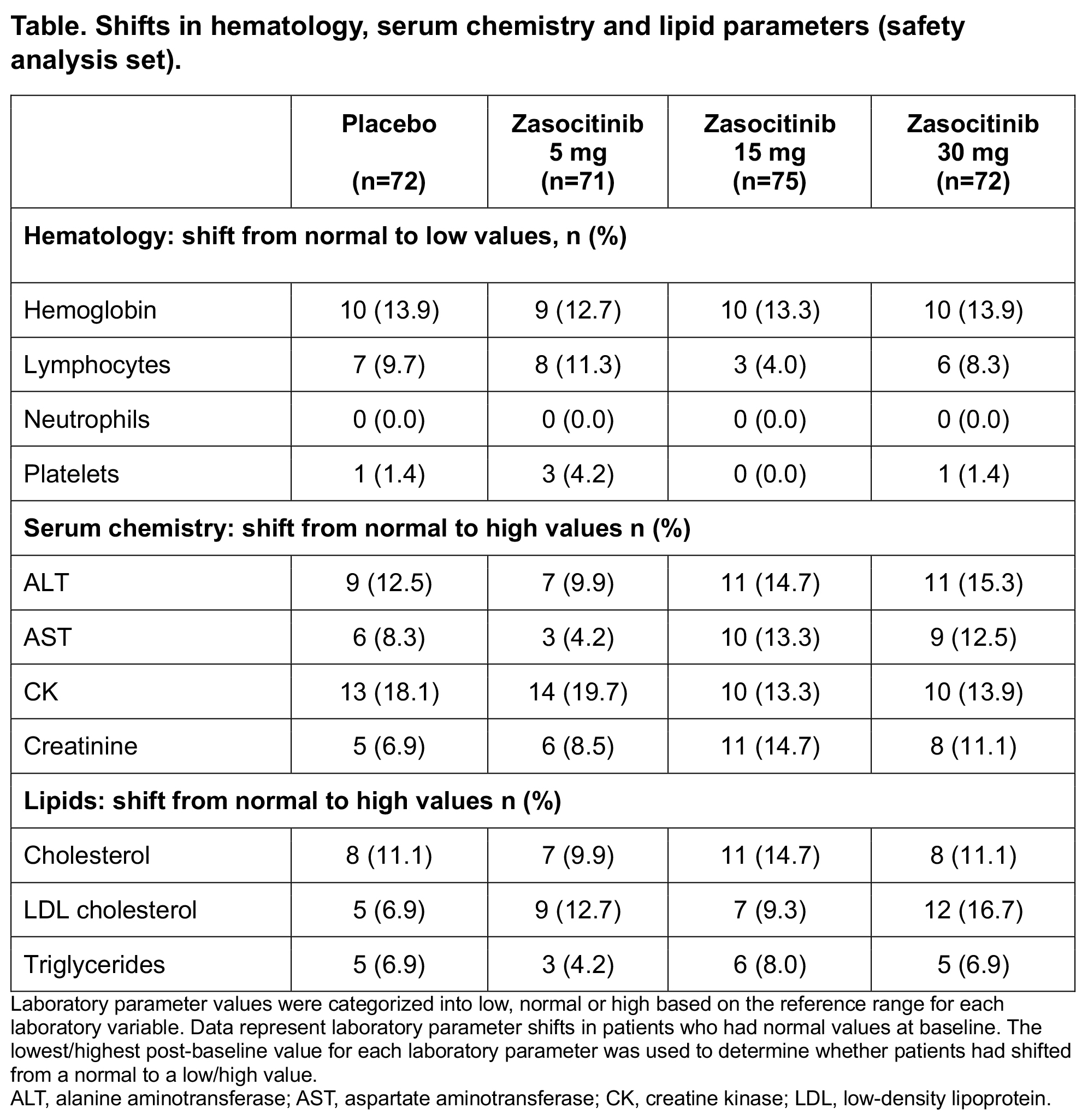
Results: In total, 290 patients were included in this analysis. Overall, 62.4% of patients were on conventional DMARDs at baseline (57.6% on methotrexate). Laboratory parameters generally stayed within the normal range for most patients, with no clinically meaningful longitudinal changes observed across any treatment arms. No cases of CTCAE Grade ≥3 of neutropenia, lymphopenia or thrombocytopenia were observed in any study arm; CTCAE Grade 2 of lymphopenia (n, %) was observed: PBO, 2 (2.8%); 5mg, 4 (5.6%); 15mg, 1 (1.3%), 30mg, 2 (2.8%). Of the six patients experiencing CTCAE Grade ≥3 elevations in creatine kinase (CK), 1 (1.4%), 2 (2.8%), 3 (4.0%) and 0 (0.0%) were in the PBO and zasocitinib 5mg, 15mg and 30mg groups, respectively; only one event was drug-related and all CK elevations were resolved without any discontinuations. Three patients had CTCAE Grade 2 elevations in cholesterol (one patient each from the zasocitinib 5mg, 15mg and 30mg groups); no CTCAE grade 3 elevations were reported. Overall, 20 patients had CTCAE Grade 2 elevations in triglycerides; 12 at baseline (PBO, 4 [5.6%]; 5mg, 3 [4.2%]; 15mg, 3 [4.0%]; 30mg, 2 [2.8%]) and eight at week 12 (PBO, 3 [4.2%]; 5mg, 2 [2.8%]; 15mg, 1 [1.3%]; 30mg, 2 [2.8%]); six patients had CTCAE Grade 3 elevations (PBO, 4 [1.4%]; 5mg, 1 [0.3%]; 15mg, 0 [0.0%]; 30mg, 1 [0.3%]). Shifts from normal to low or high values in the parameters evaluated occurred at generally similar rates across treatment arms including PBO, with no clear dose dependency in the zasocitinib groups (Table).
Conclusion: Treatment with zasocitinib did not appear to result in laboratory parameter changes reported in studies of JAK1/2/3 inhibitors. Further larger phase 3 studies in patients with active PsA are planned to confirm these observations.
References
1. Kivitz A et al. Arthritis Rheumatol. 2023; 75 (suppl 9).
To cite this abstract in AMA style:
Kavanaugh A, Muensterman E, Lertratanakul A, Hong T, Chen J, Pothula P, Mark E, Kivitz A. Assessment of Laboratory Parameter Changes in a Phase 2b Trial of Zasocitinib (TAK-279), an Oral, Highly Selective TYK2 Inhibitor, in Patients with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/assessment-of-laboratory-parameter-changes-in-a-phase-2b-trial-of-zasocitinib-tak-279-an-oral-highly-selective-tyk2-inhibitor-in-patients-with-active-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-laboratory-parameter-changes-in-a-phase-2b-trial-of-zasocitinib-tak-279-an-oral-highly-selective-tyk2-inhibitor-in-patients-with-active-psoriatic-arthritis/