Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: PsA, a chronic systemic
inflammatory disease, reduces physical function and QOL. Treatment improves/maintains
functionality. PALACE 1-3
compared apremilast (APR) efficacy/safety with placebo (PBO) in patients (pts)
with active PsA despite prior conventional DMARDs and/or biologics, providing
one of the largest databases (N=1,489) examining physical disability in
moderate to severe PsA pts. The impact of APR 30 mg BID (APR30) treatment over
104 wks on disability was assessed using the HAQ-DI in a pooled analysis of the
PALACE 1-3 database.
Methods:
Pts were randomized (1:1:1)
to PBO, APR30, or APR 20 mg BID (APR20) stratified by baseline (BL) DMARD use (yes/no).
At Wk 24, all remaining PBO pts were re-randomized to APR30 or APR20. HAQ-DI scores
were collected at BL and Wks 16, 24, 40, 52 65, 78, 91, and 104. Data are analyzed
by ITT, LOCF methodology for Wk 16, and described as data as observed to Wk 104,
with LOCF analysis done to confirm results. HAQ-DI MCID
decreases ≥0.13 and ≥0.30 were prespecified; a post hoc analysis for
the most recently published MCID ≥0.35 was done. Disability categories were
calculated using HAQ-DI cutoff levels ≤1.0 (clinically significant disability1)
and ≤0.5 (MDA criteria2); assessments included proportion
reaching levels ≤0.25. Shift categories of scores were
examined with increments of 0.25 to clarify pt disability level and category shifts.
Results: Pts exhibited significant physical
disability at BL (mean HAQ-DI=1.2); 60% of APR30 pts had >1.0, and 31% had >1.5,
noting marked difficulty/need for assistive devices in performing activities of
daily living. Major disability was noted in up to 13% with BL HAQ-DI >1.875.
As early as Wk 16, physical function improved with APR30; pts exhibited a mean
HAQ-DI change of -0.21 (vs. -0.07 PBO; P<0.0001), 56% achieved HAQ-DI
≤1.0 at Wk 16 (vs. 48% PBO pts), and 29%
achieved HAQ-DI ≤0.5 (vs. 25% PBO pts). Fewer PBO vs.
APR30 pts reached MCID of -0.13 (37% vs. 47%; P<0.005)
and -0.30 and -0.35 (26% vs. 36%; P<0.005). At Wk 52, decreases in disability
were maintained (APR30 mean change in HAQ-DI=-0.33); 48% of APR30 pts achieved
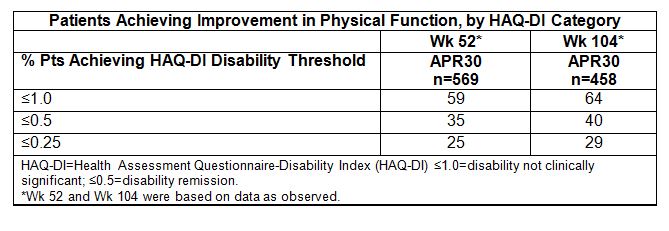
MCID -0.30 and 48% MCID -0.35. Importantly, at Wk 52, 59% of all APR30 pts achieved
HAQ-DI ≤1.0, 35% ≤0.5, and 25% ≤0.25 (Table). Among pts with greater BL
disability (HAQ-DI
≥1.5), 64% improved
by ≥1 shift category and 48% by ≥2. At Wk 104, 50% of APR30 pts achieved MCID -0.30 and
MCID -0.35, with 64% achieving HAQ-DI ≤1.0, 40% ≤0.5, and 29% ≤0.25.
Conclusion: In APR30 pts, physical function improved
and was sustained with long-term treatment. Most pts achieved HAQ-DI MCID -0.30
or -0.35 and HAQ-DI scores ≤1.0, with many obtaining HAQ-DI ≤0.5, defined
as minimal disease in recently developed criteria. These data indicate
improvement and long-term maintenance of functionality with APR treatment.
References: 1. Sokka T, et al. Arthritis Rheum.
2003;48:59-63. 2. Coates LC, et al. Ann Rheum Dis. 2010;69:48-53.
To cite this abstract in AMA style:
Mease PJ, Wollenhaupt J, Hall S, van Den Bosch F, Lespessailles E, McIlraith M, Teng L, Edwards CJ. Assessment of Disability Levels in a Cohort of 1,489 Patients with Active Psoriatic Arthritis, and the Effect of Apremilast Treatment: Pooled Data from Three Phase III, Randomized, Controlled Trials [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/assessment-of-disability-levels-in-a-cohort-of-1489-patients-with-active-psoriatic-arthritis-and-the-effect-of-apremilast-treatment-pooled-data-from-three-phase-iii-randomized-controlled-trials/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-disability-levels-in-a-cohort-of-1489-patients-with-active-psoriatic-arthritis-and-the-effect-of-apremilast-treatment-pooled-data-from-three-phase-iii-randomized-controlled-trials/