Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tyrosine kinase 2 (TYK2) is an intracellular kinase involved in key cytokine pathways linked to PsA pathophysiology. Several of these pathways are intertwined with those that mediate pain signaling and fatigue, symptoms that patients with PsA commonly cite as negatively affecting quality of life and daily activities. Deucravacitinib is a first-in-class, oral, selective, allosteric TYK2 inhibitor approved in multiple countries for the treatment of adults with plaque psoriasis. In a phase 2 trial in patients with active PsA, deucravacitinib treatment resulted in a reduction in pain and fatigue as well as the levels of key cytokines, vs placebo (PBO)1; deucravacitinib has also demonstrated efficacy vs PBO in patients with SLE receiving standard background therapy. Previous studies have shown the effects of therapeutic interventions on pain may have both direct and indirect contributions. Here, we apply a mediation analysis of data from the phase 2 PsA trial (NCT03881059) to characterize and quantify the potential direct effects of deucravacitinib on pain and fatigue vs. observable indirect effects achieved through improvements in available clinical endpoints associated with inflammation or swelling.
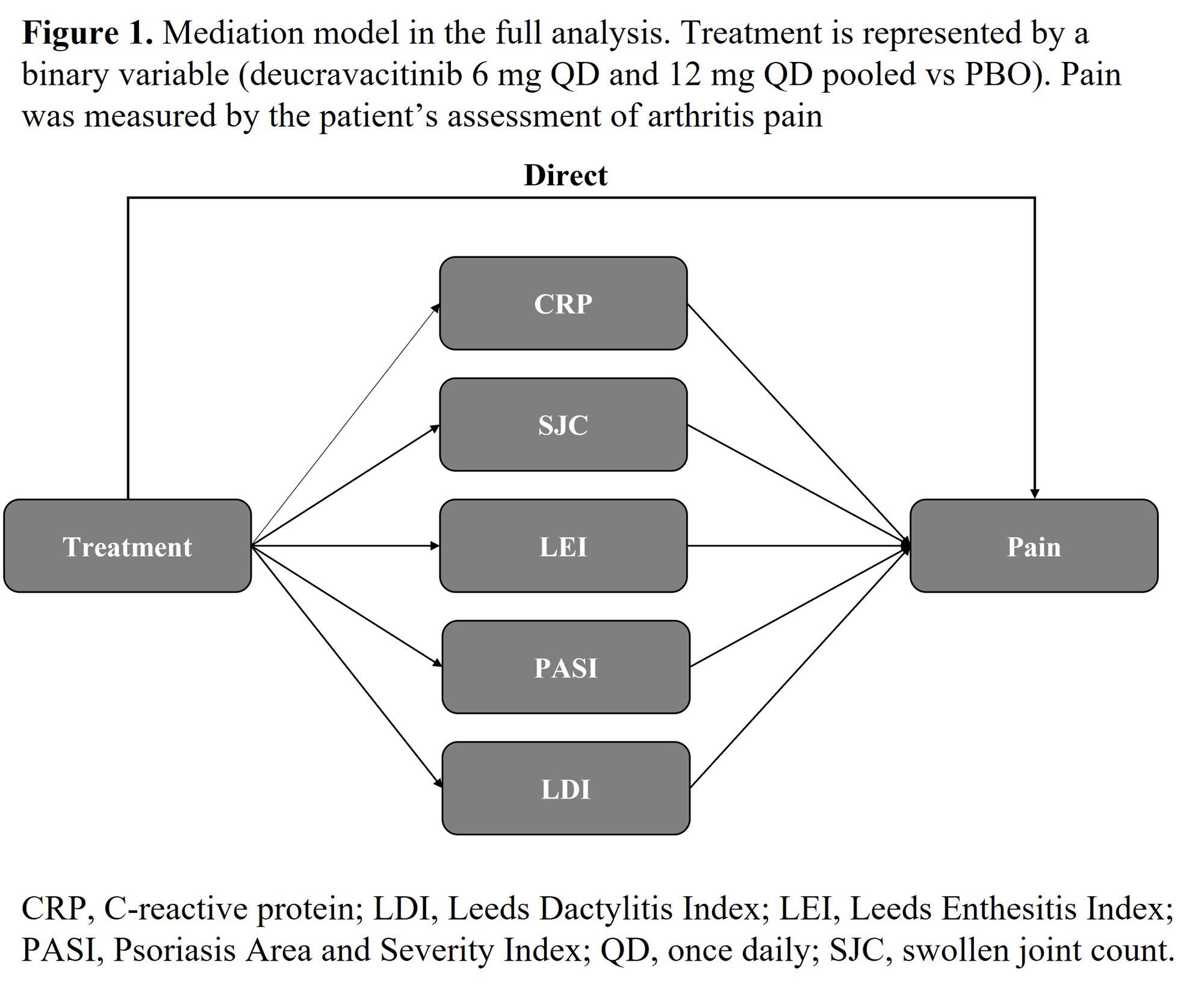
Methods: All data included are from week 16 observations in the phase 2 trial of deucravacitinib (6 mg QD and 12 mg QD pooled) v. PBO in patients (N=203) with active PsA. Improvements in pain and 5 clinical endpoints associated with inflammation or swelling were included in the initial mediation model. These endpoints included C-reactive protein (CRP), swollen joint count (SJC), Psoriasis Area and Severity Index (PASI), Leeds Enthesitis Index (LEI), and Leeds Dactylitis Index (LDI) (Figure 1). The relative contribution of each parameter to the improvement in pain was derived and used to develop a refined model including only those parameters which reached or neared statistical significance. The same methodology was repeated for fatigue.
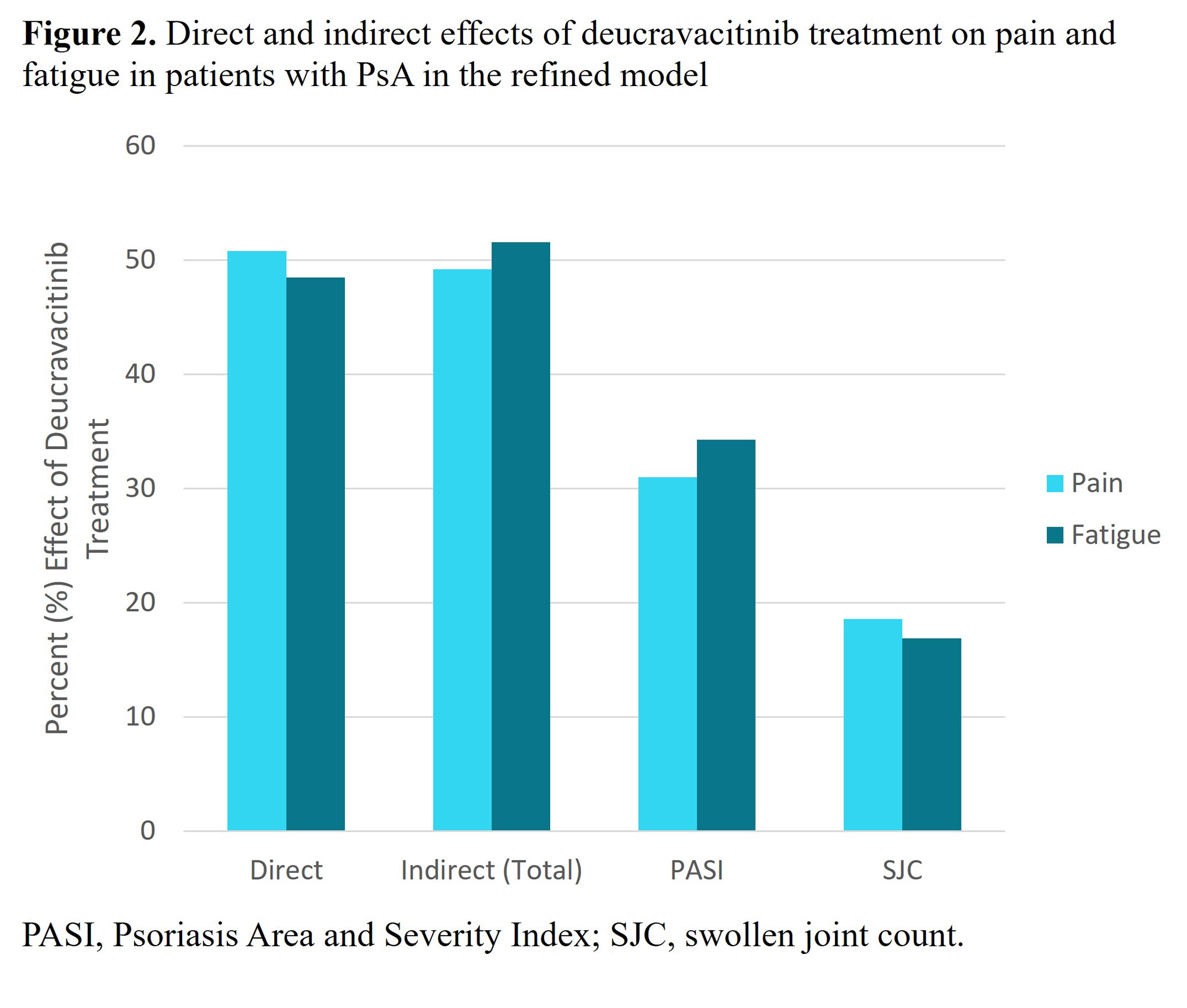
Results: In the initial model, the strongest indirect mediators of both pain and fatigue with deucravacitinib treatment were SJC and PASI (Table). The following parameters were not significantly associated with the mediation of pain or fatigue: CRP, LEI, LDI. In the refined model, the total observable indirect effects of deucravacitinib on pain and fatigue were calculated to be 49.2% and 51.5%, respectively, derived from improvements in SJC and PASI. The remaining, potentially direct, effect on pain and fatigue improvement is calculated to be 50.8% and 48.5% respectively. (Figure 2).
Conclusion: Improvements in pain and fatigue in patients with PsA treated with deucravacitinib may be attributable to both direct and indirect mechanisms. PASI- and SJC-mediated improvements in pain and fatigue contributed the most to the observed indirect effects. Further investigation into the potential direct effect of deucravacitinib on pain and fatigue are needed.
Reference: 1. Mease P, et al. Ann Rheum Dis 2022; 81:815-822.
To cite this abstract in AMA style:
Mease P, Eder L, Ogdie A, Deodhar A, Banerjee S, Jou Y, Nowak M, Lehman T, Strand V. Assessment of Direct and Indirect Impact on Pain and Fatigue Outcomes in a Phase 2 Clinical Trial of Deucravacitinib, a Selective, Allosteric Tyrosine Kinase 2 Inhibitor, in Patients with Active PsA: A Mediation Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/assessment-of-direct-and-indirect-impact-on-pain-and-fatigue-outcomes-in-a-phase-2-clinical-trial-of-deucravacitinib-a-selective-allosteric-tyrosine-kinase-2-inhibitor-in-patients-with-active-psa/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-direct-and-indirect-impact-on-pain-and-fatigue-outcomes-in-a-phase-2-clinical-trial-of-deucravacitinib-a-selective-allosteric-tyrosine-kinase-2-inhibitor-in-patients-with-active-psa/