Session Information
Date: Saturday, November 12, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Tocilizumab (TCZ) is the only biologic drug approved in giant cell arteritis (GCA), based in two clinical trials (CT) (1,2). CT included selected patients who may differ from those of clinical practice (CP). A high proportion of GCA patients treated with TCZ in CT had a newly diagnosed GCA, whereas in CP, most of them are refractory/recurrent GCA (3,4). Although in CT the efficacy of TCZ seems to be similar in patients with newly diagnosed GCA and in patients with refractory/recurrent GCA, in CP it is not documented.
The purpose of this study is to compare in CP, the effectiveness and safety of TCZ in newly diagnosed vs refractory/recurrent GCA.
Methods: Multicentre observational study on 471 GCA patients treated with TCZ. GCA was diagnosed by: a) ACR criteria, and/or b) temporal artery biopsy, and/or c) imaging techniques.A comparative study between patients with newly diagnosed GCA (< 6 weeks) and those with refractory/recurrent GCA ( >6 weeks) (according to GiACTA study definitions) (2). Sustained remission was based on EULAR definitions (5).
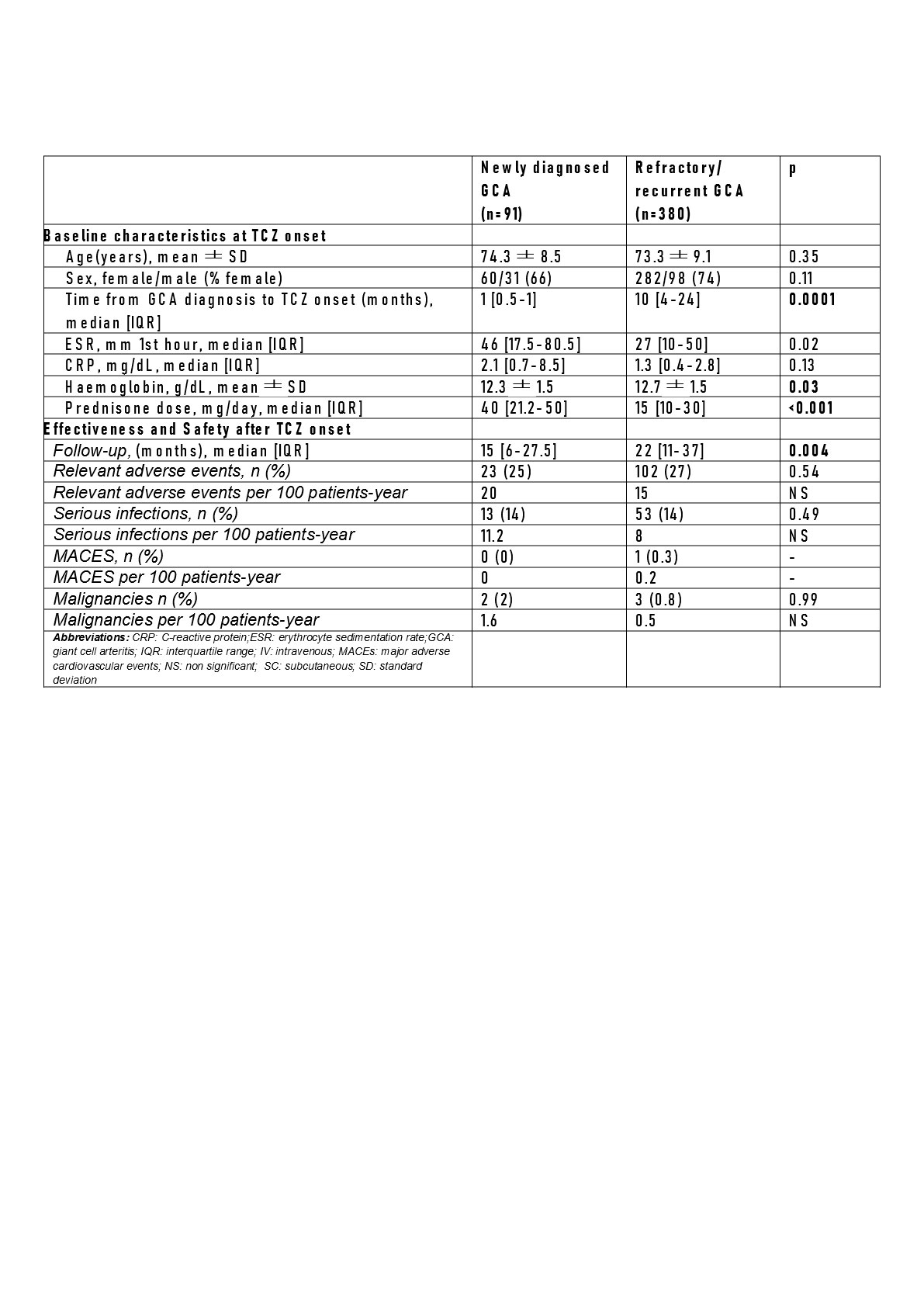
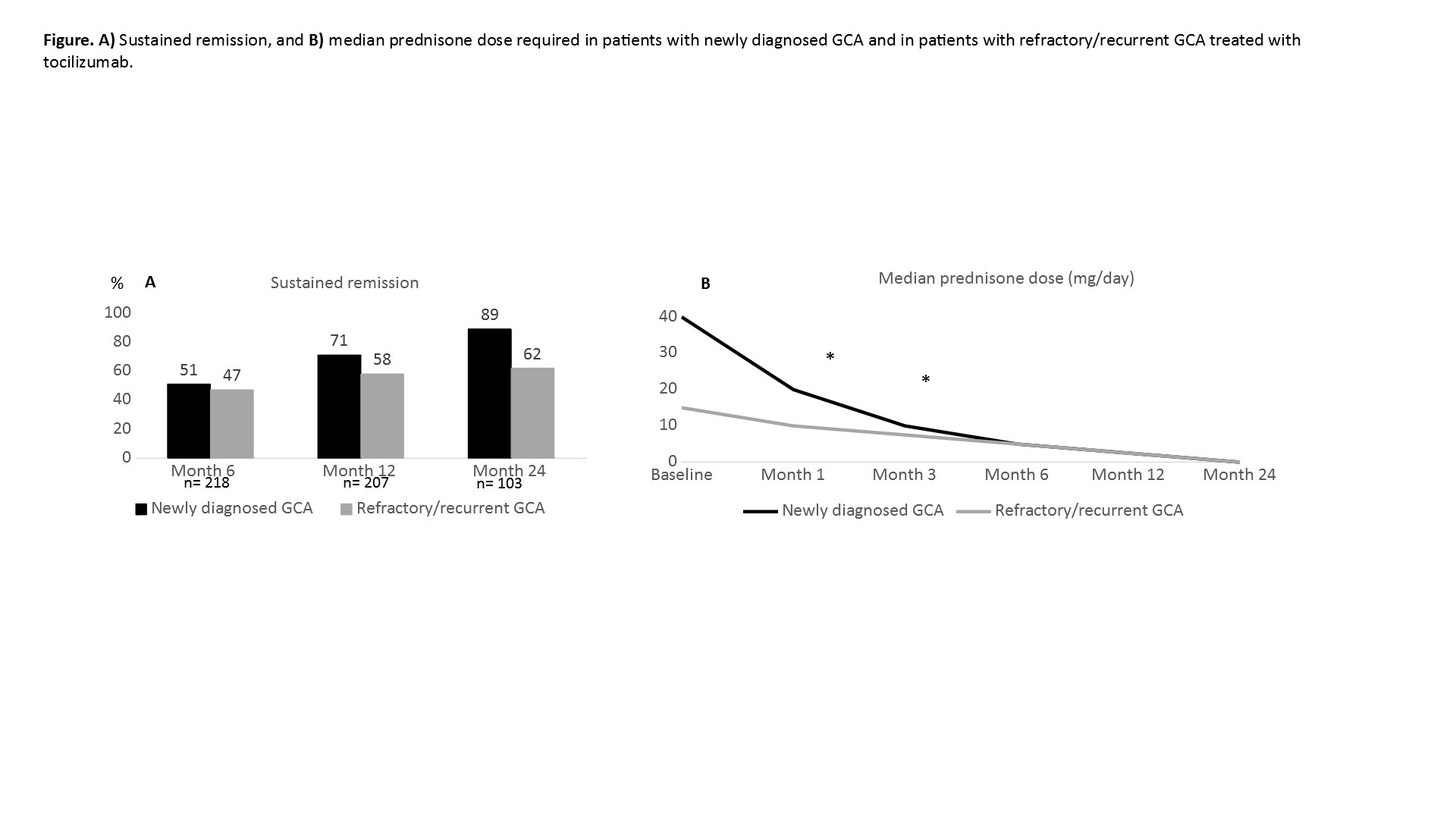
Results: The 471 GCA patients were divided into 2 subgroups: a) newly diagnosed GCA (n=91) and b) refractory/recurrent GCA (n=380) (TABLE).No significant differences were observed between both groups in sustained remission, although a greater tendency towards sustained remission is observed in newly diagnosed than in refractory/recurrent GCA patients (FIGURE). The decrease in glucocorticoids dose was faster in the first three months in the newly diagnosed GCA group, but thereafter, was similar in both groups, as well as the appearance of relevant adverse events and serious infections.
Conclusion: The effectiveness and safety of TCZ seems to be similar in patients with newly diagnosed GCA and in patients with refractory/recurrent GCA.
References
1. Villiger PM, et al. Lancet. 2016; 387:1921-1927. PMID: 26952547
2. Stone JH, et al. N Engl J Med. 2017; 377:317-328. PMID: 28745999
3. Calderón-Goercke M, et al. Semin Arthritis Rheum. 2019; 49: 126-135. PMID: 30655091
4. Calderón-Goercke M, et al. Clin Exp Rheumatol. 2020; 124: S112-119. PMID: 32441643
5. Hellmich B, et al. Ann Rheum Dis. 2020; 79: 19-30. PMID: 31270110
To cite this abstract in AMA style:
Sánchez-Martín J, Loricera J, Moriano C, Castañeda S, Narváez F, Aldasoro V, Maiz O, Melero R, Villa Blanco J, Vela-Casasempere P, Romero Yuste S, Callejas J, De Miguel E, Galíndez-Agirregoikoa E, Sivera F, Fernandez J, Galisteo C, Ferraz Amaro I, Sánchez-Bilbao L, Calderon-Goercke M, González-Gay M, Blanco R. Assessing the Effectiveness of Tocilizumab in Newly Diagnosed Giant Cell Arteritis versus Refractory/recurrent Giant Cell Arteritis in Clinical Practice [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/assessing-the-effectiveness-of-tocilizumab-in-newly-diagnosed-giant-cell-arteritis-versus-refractory-recurrent-giant-cell-arteritis-in-clinical-practice/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-the-effectiveness-of-tocilizumab-in-newly-diagnosed-giant-cell-arteritis-versus-refractory-recurrent-giant-cell-arteritis-in-clinical-practice/