Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Neonatal lupus (NL), driven by fetal exposure to maternal anti-SSA/Ro autoantibodies, typically results in permanent cardiac manifestations in utero and/or a transient rash postnatally. Considering the contribution of fibrosis to the pathogenesis of organ injury and implications with regard to therapeutic approach in NL, this study was initiated to investigate molecular underpins of fetal cardiac and neonatal dermal fibroblasts in the context of maternal anti-Ro-propagated injury.
Methods: In an in vitro model of anti-Ro-initiated scarring, 2nd trimester human fetal cardiac fibroblasts or neonatal dermal fibroblasts were co-cultured with supernatants (sup) from THP-1 macrophages transfected with hY3 (noncoding ssRNA and TLR 7/8 agonist binding Ro60), TGF-β or IFNα for 24-72 hrs. Relative fibroblast heterogeneity was confirmed through the expression of S100 Calcium-Binding Protein A4 (S100A4) and/or myofibroblast marker α-SMA. Functional fibroblast readouts—cell migration and wound closure—were assessed by scratch assay.
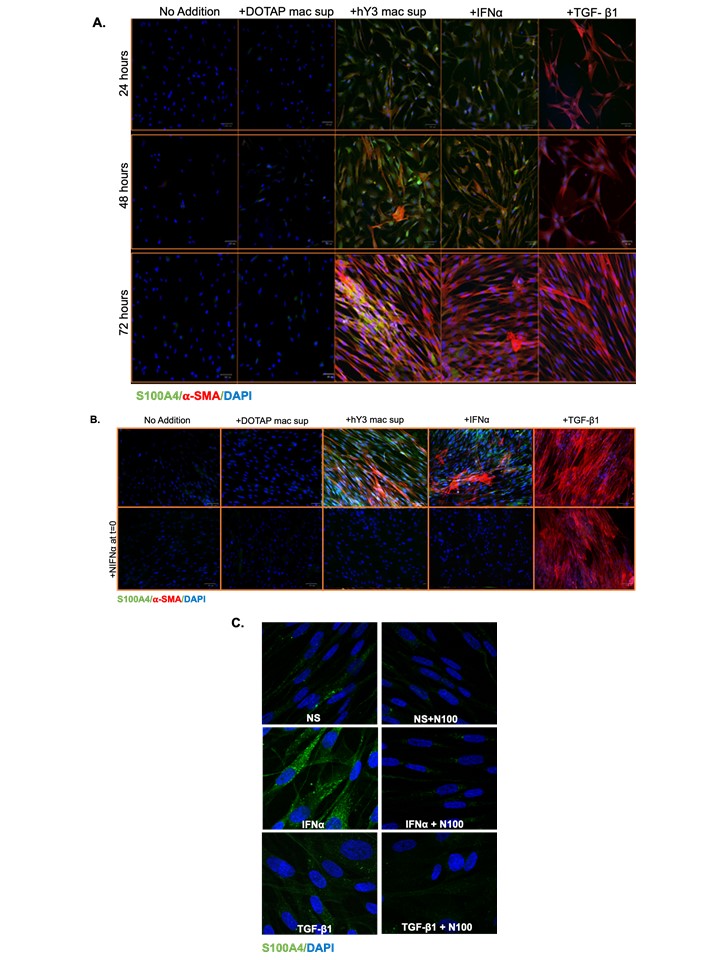
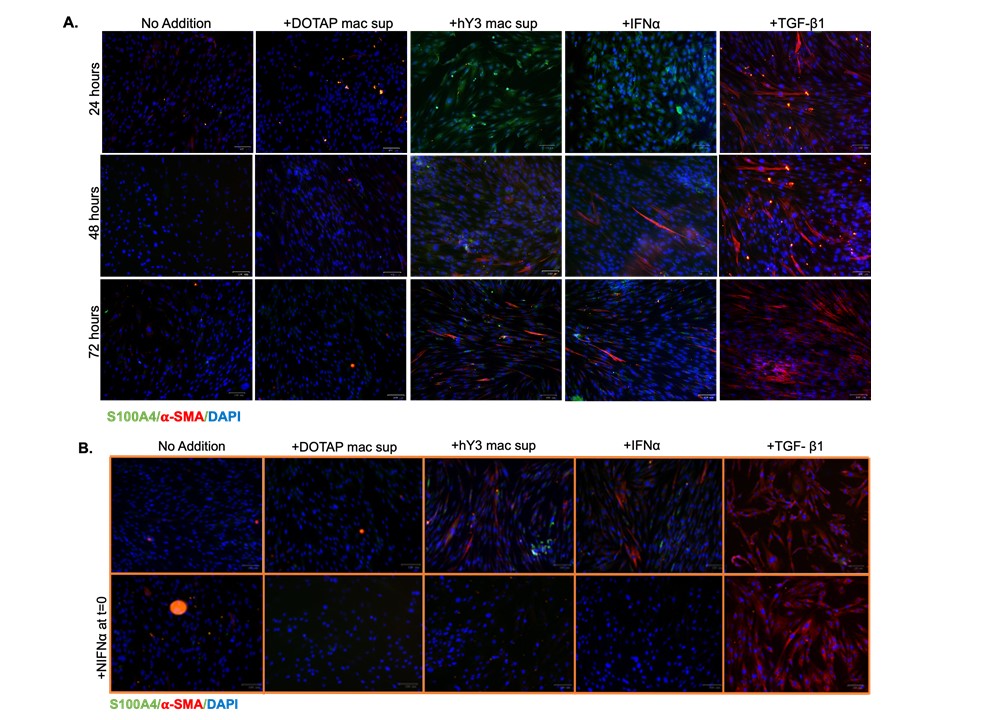
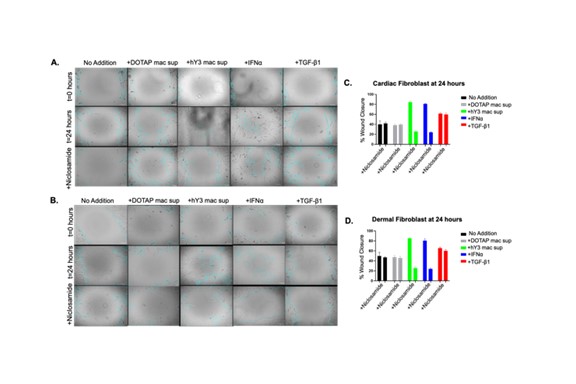
Results: Immunofluorescence evaluated 24, 48, and 72 hrs after treatment (DOTAP sup, hY3 sup or IFNα or TGF-β1) established a time-course of S100A4 and α-SMA expression in cardiac fibroblasts (Fig 1A) and dermal fibroblasts (Fig 2A). At 24 hrs following the addition of hY3 sup or IFNα, S100A4 expression predominated in both cell types. By 48 hrs, cardiac and dermal fibroblasts showed increased α-SMA expression, primarily through the emergence of a dual-positive (α-SMA+ S100A4+) subpopulation (Fig 1A). At 72 hrs, three subpopulations (S100A4+, α-SMA+, and dual α-SMA+S100A4+) were observed in fetal cardiac fibroblasts treated with hY3 sup or IFNα, with the α-SMA+ population being most prominent (Fig 1A). In contrast, the proportion of α-SMA+ myofibroblasts was markedly diminished in the dermal fibroblasts at 72 hrs (Fig 2). Neutralizing antibodies to IFNα inhibited α-SMA and S100A4 expression in cardiac and dermal fibroblasts cultured with hY3 sup, validating type I IFN as a dominant cytokine (Fig 1B and Fig 2B). Inhibition of S100A4 by niclosamide markedly attenuated S100A4 expression after IFNα treatment of the cardiac fibroblasts (Fig 1C). Turning to fibroblast function, hY3 sup and/or IFNα resulted in rapid wound closure after 24 hrs as indicated by a smaller gap area in the cardiac fibroblasts (Fig 3A and C) and in dermal fibroblasts (Fig 3B and D). Niclosamide mitigated the wound area closure after 24 hrs in both cardiac and dermal fibroblasts cultured with hY3 sup or IFNα (Fig 3).
Conclusion: These results suggest that in NL, the initial macrophage-fibroblast injury mediated by anti-Ro is driven by macrophage secreted type I IFN. Resultant S100A4 expression triggers cardiac fibroblasts to transdifferentiate into myofibroblasts which is not sustained in dermal fibroblasts, consistent with the transient clinical phenotype of cutaneous NL. Pathologic cardiac and dermal fibroblast migration appears dependent largely on S100A4 rather than α-SMA. Niclosamide treatment effectively inhibits fibroblast migratory and profibrotic functions, suggesting a role in the therapeutic approach to NL which may be applicable to other organ injury.
To cite this abstract in AMA style:
Sachan N, Firl C, Carlucci P, Fraser N, Clancy R, Buyon J. Assessing S100A4 and Myofibroblast Phenotypes in the Pathogenesis of Cardiac and Cutaneous Neonatal Lupus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/assessing-s100a4-and-myofibroblast-phenotypes-in-the-pathogenesis-of-cardiac-and-cutaneous-neonatal-lupus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-s100a4-and-myofibroblast-phenotypes-in-the-pathogenesis-of-cardiac-and-cutaneous-neonatal-lupus/