Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Spondyloarthritis Including PsA – Treatment I: Axial Spondyloarthritis
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Since the last update of the ASAS-EULAR recommendations for the management of axial spondyloarthritis (axSpA) in 2016, substantial new evidence has become available on the management of axSpA. Our aim was to update the ASAS-EULAR recommendations for the management of axSpA.
Methods: Following the EULAR Standardised Operating Procedures, two systematic literature reviews were conducted on non-pharmacological and pharmacological treatment of axSpA. During a task force meeting the evidence was presented, discussed and overarching principles and recommendations were updated, followed by voting.
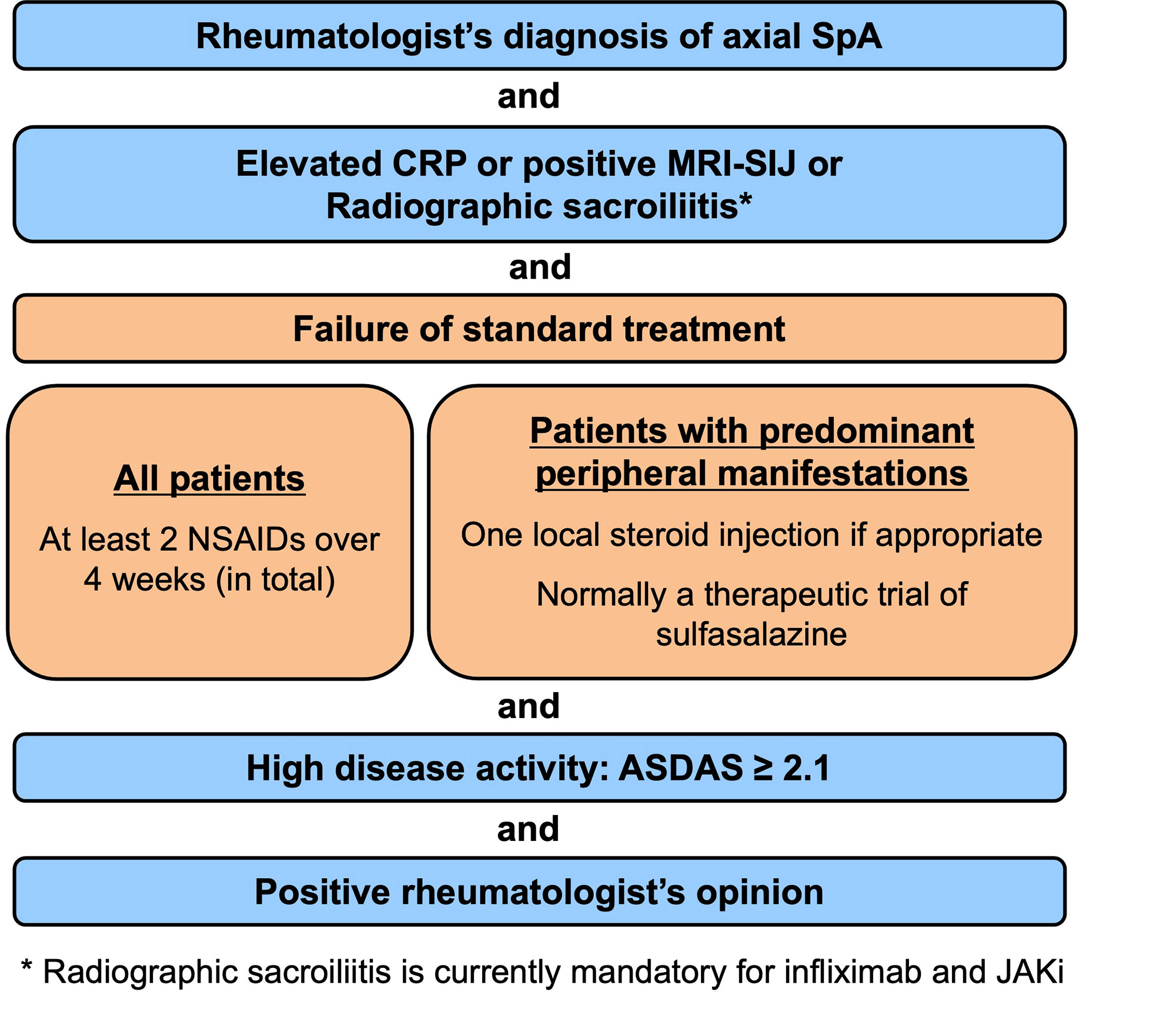
Results: A total of 5 overarching principles (unchanged compared to the previous version of the recommendations) and 15 recommendations were formulated (Table). All recommendations included in the previous version were kept: eight unchanged (#2,3,6,7,8,13,14,15); three with minor edits, mostly on nomenclature (#1,4,5) and two with relevant updates (#9,12), while two newly formulated recommendations (#10,11) were added. The first 5 recommendations focus on personalised medicine, including treatment target and monitoring, non-pharmacological management and non-steroidal anti-inflammatory drugs (NSAIDs) as first choice pharmacological treatment. Recommendations 6-8 deal with analgesics and discourage long-term glucocorticoids and conventional synthetic DMARDs for pure axial involvement. Recommendation 9 describes the indication for biological DMARDs (bDMARDs i.e. TNFi, IL-17i) and this was expanded to targeted synthetic DMARDs (tsDMARDs i.e. JAKi). b/tsDMARDs are indicated for patients with elevated CRP, MRI inflammation of SI joints or radiographic sacroiliitis who have high disease activity (ASDAS≥2.1) and failed ≥2 NSAIDs (Figure). BASDAI is no longer recommended to assess treatment start. Current practice is to start a TNFi or IL-17i as there is more accumulated evidence, particularly on safety, and experience with these drug classes. The continuation of a b/tsDMARD should be considered if an improvement of ASDAS≥1.1 has been achieved after ≥12 weeks. The new recommendation 10 addresses extra-musculoskeletal manifestations, with TNF monoclonal antibodies preferred for recurrent uveitis or inflammatory bowel disease, and IL-17i for significant psoriasis. In light of overdiagnosis and overtreatment, treatment failure should trigger re-evaluation of the diagnosis and consideration of the presence of comorbidities (#11 – new). If active axSpA is confirmed after failing a b/tsDMARD, switching to another b/tsDMARD is recommended (#12). Tapering, but not immediate discontinuation of a bDMARD, can be considered in patients in sustained remission (#13). The unchanged recommendations #14 and #15 deal with surgery and spinal fractures.
Conclusion: The 2022 ASAS-EULAR recommendations provide up-to-date guidance on the management of patients with axSpA.
To cite this abstract in AMA style:
Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, Landewé R, Van den bosch F, Boteva B, Bremander A, Carron P, Ciurea A, van Gaalen f, Geher P, Gensler L, Hermann J, de Hooge M, Husakova M, Kiltz U, Lopez-Medina C, Machado P, Marzo-Ortega H, Molto A, Navarro-Compán V, Nissen M, Pimentel-Santos F, Poddubnyy D, Proft F, Rudwaleit M, Telkman M, Zhao S, Ziade N, van der Heijde D. ASAS-EULAR Recommendations for the Management of Axial Spondyloarthritis: 2022 Update [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/asas-eular-recommendations-for-the-management-of-axial-spondyloarthritis-2022-update/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/asas-eular-recommendations-for-the-management-of-axial-spondyloarthritis-2022-update/