Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Fibroblast-like synoviocytes (FLS) play major roles on joint destruction in rheumatoid arthritis (RA) through migrating and invasing cartilage and bone by secreting proteases such as matrix metalloproteinases (MMPs).Recent studies show that artesunate, an important artemisinin derivative, may inhibit proinflammatory cytokines secretion of RA-FLS. We aim to investigate the effect of artesunate on migration and invasion of RA-FLS and itsunderlying mechanism
Methods: FLS isolated from knee synovium of active RA patients were cultured in vitro. The effects of artesunate, methotrexate (MTX) or hydroxychloroquine (HCQ) on cell viability and anti-proliferation were measured by CCK-8 assay. Effects on migration and invasion capacity were detected by wound healing and transwell assays. Differential expression of MMPs were analyzed by Proteome profiler human protease array (R&D Systems) and furtherly verifiedby quantitative real-time PCR, western blot (WB) and ELISA. The expression of PI3K, PIP2, PIP3, PDK1 and Akt in PI3K/Akt signal pathway was measured by WB.
Results: The IC50 value of artesunate, MTX and HCQ on RA-FLS were 6891μM, 181.4nM and 5433μM respectively. Compared with untreated group, IC1-20μM, IC3-40μM, IC5-60μM of artesunate, IC5-10nM of MTX and IC5-20μM of HCQ showed no significant change in proliferation even after 72h. Wound healing and migration assays for 12h and invasion assay for 24h showed artesunate inhabits the migration and invasion of RA-FLS in a dose-dependent manner. MTX also has inhibition effect on the migration and invasion of RA-FLS, but HCQ not (Fig. A). Proteome profiler human protease array of culture supernatant showed that 60μM artesunate markedly inhibited MMP-2/9 expression (Fig. B). Further verification showed that RA-FLS pretreated with 60μM artesunate attenuated MMP-2/9 mRNA and protein expression, and ELISA results showed 60μM artesunate decreased MMP-2 and MMP-9 concentration of 5.82±0.45 and 11.74±1.30ng/mL respectively (Fig. C). Further pre-treatment with recombination human MMP-2 (6 ng/ml) or MMP-9 (12 ng/ml) for 24h could reverse inhibitory effect of 60μM artesunate on invasion, but not migration (Fig. D). Quantitative real-time PCR and WB analysis showed that PDK1 expression and Akt activity (phophso-Akt/Akt) in 60μM artesunate treatment group was significantly lower than that in untreated group which indicated that artesunate suppressed generation of PDK1-induced activation of Akt (Fig. E).
Conclusion: Artesunate could inhibit migration and invasion of RA-FLS and MMP-2/9 expression through suppressing PDK1-induced activation of Akt.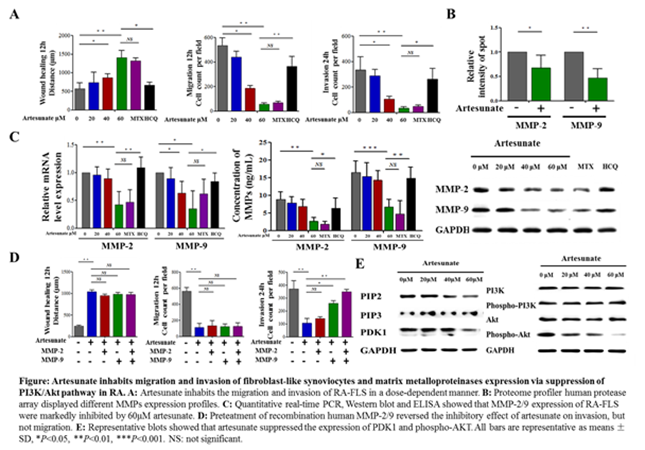
To cite this abstract in AMA style:
Ma JD, Jing J, Yan T, Mo YQ, Dai L. Artesunate Inhabits Migration and Invasion of Fibroblast-like Synoviocytes and Matrix Metalloproteinases Expression Via Suppression of PI3K/Akt Pathway in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/artesunate-inhabits-migration-and-invasion-of-fibroblast-like-synoviocytes-and-matrix-metalloproteinases-expression-via-suppression-of-pi3kakt-pathway-in-rheumatoid-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/artesunate-inhabits-migration-and-invasion-of-fibroblast-like-synoviocytes-and-matrix-metalloproteinases-expression-via-suppression-of-pi3kakt-pathway-in-rheumatoid-arthritis/
