Session Information
Date: Tuesday, November 7, 2017
Title: Rheumatoid Arthritis – Clinical Aspects IV: Medications and Risk
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Methotrexate (MTX) is a first line drug for Rheumatoid Arthritis (RA) that is associated with elevated liver function tests (LFTs) in 10- 20% of patients though only 2-3 % of patients stop MTX for this side effect. Current guidelines advise LFT testing every 2 to 3 months with little data to support the utility of such monitoring. Our goal was to describe changes made to MTX dosing in response to elevated aspartate aminotransferase (AST) levels and define any correlation between elevated AST to MTX dose and duration of therapy.
Methods: The electronic health records of RA patients on MTX from 7/1/2009 to 12/31/2015 were reviewed for elevated AST values (>46 U/L),) which were categorized as mild ( >1- < 2x upper limits of normal,ULN) or severe (³2x ULN). We recorded the MTX dose and duration, and action taken on the dose (continued, decreased, stopped, or stopped for other reason than MTX) at the time of the AST elevation. The last AST observed for each patient while on MTX was used for analysis. Fisher’s Exact test was used to compare the proportions of actions taken in each AST category. A Spearman correlation was used to assess the correlation between MTX dose and AST levels; t-tests were used to compare MTX duration between the AST levels.
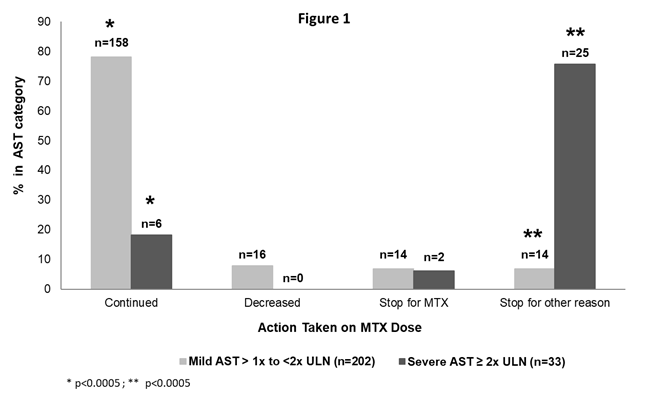
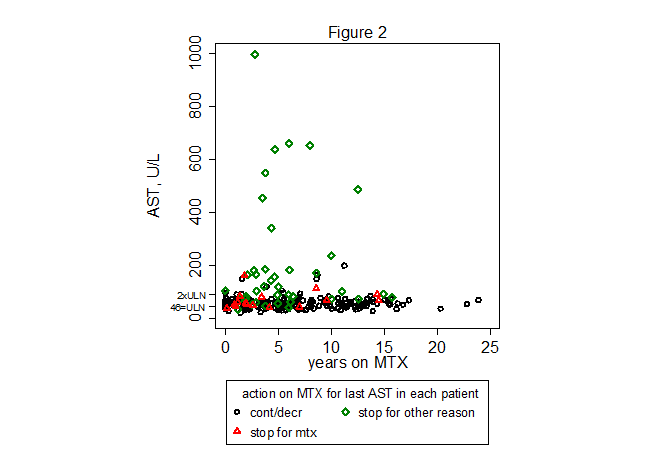
Results: Of 1158 RA patients taking MTX, 235 (20.2%) had at least one elevated AST value. Sixteen (6.8%) patients with elevated AST stopped MTX, because of provider concern for hepatotoxicity from the drug. Most patients (78%) with mild AST elevation continued MTX (p<0.0005), although 18% of patients continued MTX despite severe AST elevation (Figure 1). Providers stopped MTX for other reasons (infection, malignancy, cardiac event, cholelithiasis) in 76% of patients with severe AST elevation. There was no correlation between MTX dose and elevated AST (Spearman correlation = -0.015). Patients with mild AST elevation were on MTX for a mean (SD) of 73.3 (60.7) months which was no different from those with severe AST elevation at 69.5 (47.2) months, p=0.736 (Figure 2, AST level vs MTX duration).
Conclusion: Liver enzyme elevation, as measured by AST level, rarely resulted in MTX cessation for suspected MTX toxicity. The most common reason for stopping MTX was for AST elevation in the presence of an acute illness not related to RA. We advocate less frequent LFT testing for patients on MTX when the patient is clinically stable due to lack of evidence that monitoring leads to meaningful change in provider prescribing practice.
To cite this abstract in AMA style:
Lau CC, Sayeed SM, Kennedy A, Skelly J, Cooper S. Are We over-Testing for Liver Enzyme Abnormalities in Rheumatoid Arthritis Patients Prescribed Methotrexate? [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/are-we-over-testing-for-liver-enzyme-abnormalities-in-rheumatoid-arthritis-patients-prescribed-methotrexate/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/are-we-over-testing-for-liver-enzyme-abnormalities-in-rheumatoid-arthritis-patients-prescribed-methotrexate/