Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Although well tolerated by most patients, hydroxychloroquine (HCQ) can cause irreversible retinal damage. The American Association of Ophthalmology (AAO) 2016 Guidelines recommend a baseline eye exam and then annual exam after 5 years of HCQ use in patients without risk factors for retinopathy. In this study, we evaluated whether patients who are receiving HCQ were counseled to receive screening for HCQ-induced retinal toxicity and whether screening procedures were congruent with AAO Guidelines.
Methods: We analyzed system-wide electronic health records (EHR) from a large, academic health center to identify 704 patients with a face-to-face encounter in which HCQ was prescribed between 1/1/2014- 3/14/2016. A random sample of 200 patients was chosen for further analysis. 19 patients were excluded because they were not taking HCQ or did not have >1 visit with a rheumatology or dermatology clinician based on chart review. Baseline characteristics of the patients were identified through queries of structured EHR fields. Physician chart review was used to determine if counseling on the need for retinopathy screening was documented and if patients had seen an ophthalmologist. Chi-square tests were used to determine if there was a significant difference in recommended retinopathy screening intervals based on clinical department. Among the subset of patients seen by an ophthalmologist within our system, we determined whether tests were performed according to AAO guidelines.
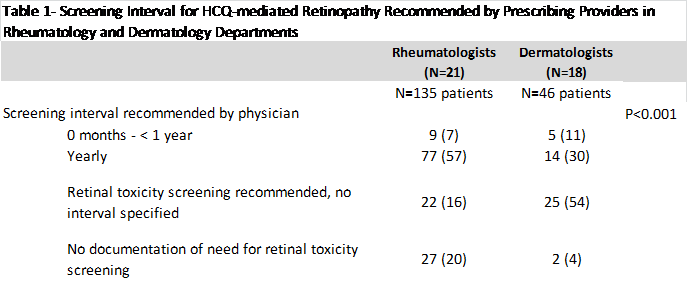
Results: Of the 181 patients included in the study, 82% were female, the mean age was 40±18.7 years, 43% were Caucasian, 23% were Asian, 17% Hispanic, 5% African American, and 12% other/multiple. The mean Charlson score was 2.1±2.6 and the majority (63%) had private insurance. 152 (84%) of the patients were counseled to undergo screening for HCQ-mediated retinopathy. Table 1 lists the screening intervals recommended by clinicians, which varied significantly by department. Interestingly, of the 69 incident HCQ users, only 2 were counseled to delay annual ophthalmologic exams until 5 years of HCQ use, the recommended interval for new, low-risk users per the AAO guidelines. 115 (64%) patients saw an ophthalmologist for retinopathy screening within the study period. Of the 48 patients who were seen by ophthalmologists in our system, 25 (51%) received the more sensitive screening tests now recommended in the AAO Guidelines.
Conclusion: In our study, a majority of patients were counseled by clinicians to receive screening for HCQ-mediated retinopathy. However, very few clinicians recommended the screening interval established by the AAO 2016 Guidelines for incident users, with most suggesting yearly screening regardless of duration of use. Additionally, many patients did not receive appropriate screening for HCQ-mediated retinopathy, despite the fact that it was recommended, highlighting an area for future quality improvement efforts.
To cite this abstract in AMA style:
Haserodt S, Tonner C, Schmajuk G, Yazdany J. Are Providers Recommending Appropriate Screening for Hydroxychloroquine-Induced Retinal Toxicity to Their Patients? [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/are-providers-recommending-appropriate-screening-for-hydroxychloroquine-induced-retinal-toxicity-to-their-patients/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/are-providers-recommending-appropriate-screening-for-hydroxychloroquine-induced-retinal-toxicity-to-their-patients/