Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Most clinical trials of Psoriatic Arthritis (PsA) exclude patients with early oligoarticular (oligo) disease and there is limited evidence to drive treatment decisions in this setting. We report 48-week (wk) efficacy and safety of apremilast (APR) vs placebo (PBO) in patients with early oligo PsA.
Methods: FOREMOST (NCT03747939), a multicenter, randomized, double-blind, PBO-controlled trial (RCT), included patients with early PsA (duration ≤5 years) and limited joint involvement ( >1–≤4 swollen and >1–≤4 tender joint count [SJC and TJC]; 66–68 joints assessed; affected joints defined as sentinel). Patients were randomized 2:1 to APR (30 mg BID) or PBO for 24 wks (early escape at Wk 16), followed by an extension phase in which all patients received APR through Wk 48 (APR/APR or PBO/APR). Primary endpoint was modified minimal disease activity (MDA-Joints) in sentinel joints at Wk 16 (SJC ≤1, TJC ≤1, and 3 of the following: psoriasis Body Surface Area ≤3%, Health Assessment Questionnaire–Disability Index (HAQ–DI) ≤0.5, patient global visual analogue scale [VAS; 0-100] ≤20, patient pain VAS ≤15, Leeds Enthesitis Index ≤11). Post hoc analysis assessed (original) MDA1 (cut off: 5/7 MDA domains), mean SJC and TJC, and change in SJC/TJC over 48 wks (all joints). Exploratory outcomes (extension phase; data as observed) included MDA-Joints, clinical Disease Activity Index for PsA (cDAPSA) remission (REM ≤4) or low disease activity (LDA >4-≤13), PsA Impact of Disease 12-item questionnaire (PsAID-12) ≤4 and HAQ-DI ≤0.5 over 48 wks.
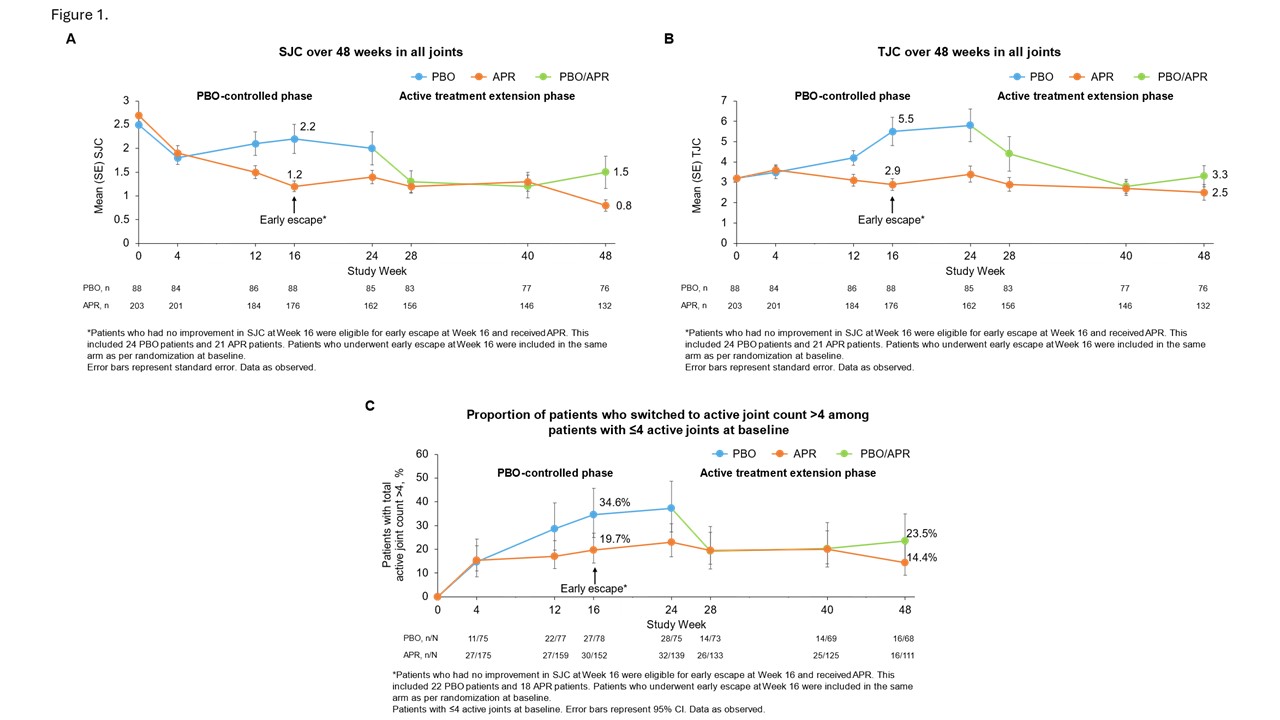
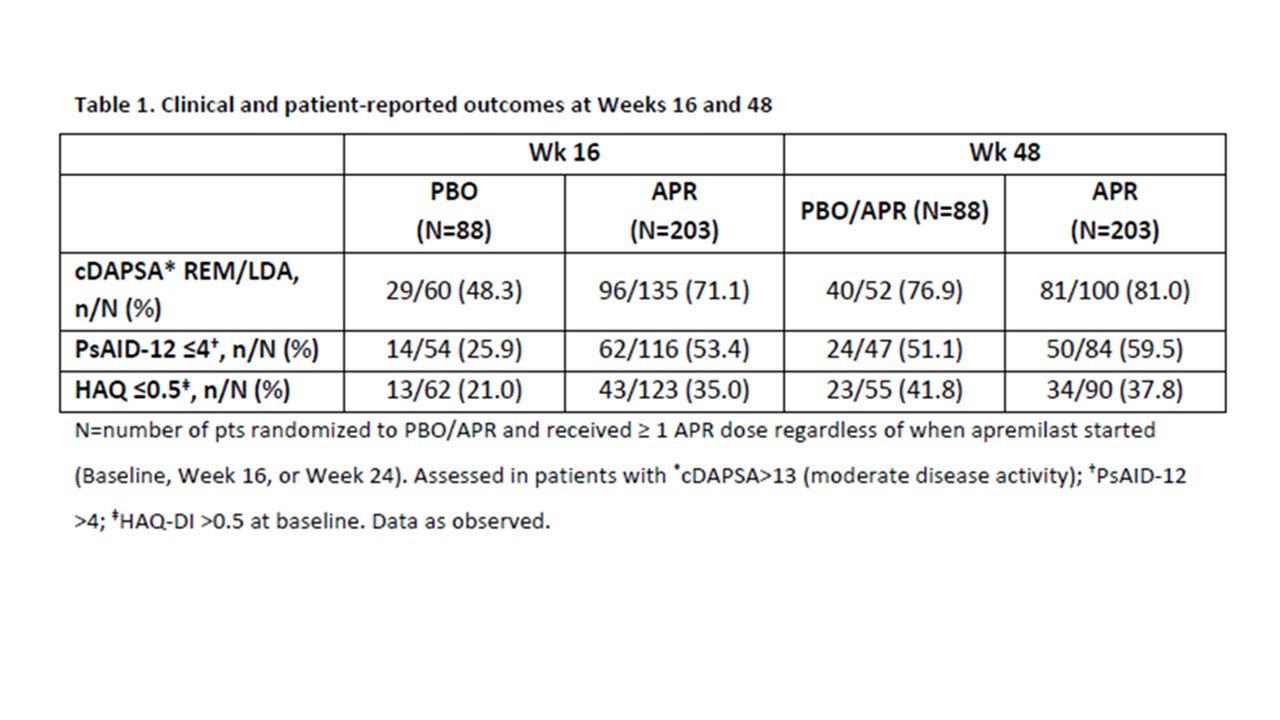
Results: In 308 randomized patients (apremilast: n=203, placebo: n=105; mean PsA duration, 9.9 months; baseline csDMARD use, 39.9%; mean SJC, 2.6; mean TJC, 3.2; mean HAQ-DI, 1.0), significantly more patients achieved MDA-Joints (primary outcome) at Wk 16 with APR vs PBO (33.9% vs 16.0%; p=0.0008)2 and MDA at Wk 16 (38.1% vs 21.0%; [nominal] p=0.0023; non-responder/multiple imputation; sentinel joints). Among patients who received ≥1 dose of APR, 46.2% of those in APR/APR and 50% in PBO/APR achieved MDA (all joints) at Wk 48. During the PBO-controlled phase, mean SJC and TJC improved in the APR group and worsened in the PBO group; further improvements were observed with APR through Wk 48 (Figure 1A-B). In patients with 2-4 sentinel joints in the PBO group, 34.6% progressed to joint count >4 at Wk 16; sustained treatment benefit was observed when these patients switched to APR, with fewer patients moving to a joint count >4 (Figure 1C). Improvements in other clinical and patient-reported outcomes observed at Wk 16 were sustained at Wk 48 with APR (Table 1), with no new safety signals.
Conclusion: In patients with early oligo PsA, APR treatment led to early improvements in clinical and patient-reported outcomes, including the treatment goal of MDA. These benefits were sustained and further improved over 48 wks with no new safety signals. To our knowledge, this is the first evidence from an RCT that can inform treatment decisions in patients with early oligo PsA.
References:
1. Coates LC, Helliwell PS. J Rheumatol. 2016;43(2):371-5.
2. Gossec L, Gladman D, Coates L, et al. Presented at: Annual Congress of the European Academy of Dermatology and Venereology; 11-14 October 2023; Berlin, Germany.
To cite this abstract in AMA style:
Gossec L, Coates L, Gladman D, Ogdie A, Wells A, Merola J, Gisondi P, pinter a, Kishimoto M, Reddy J, Amouzadeh H, Wang R, Jardon S, Brunori M, Mease P. Apremilast Treatment in Early Oligoarticular Psoriatic Arthritis Improves Clinical and Patient-Reported Outcomes for up to 48 Weeks – Data from a Phase 4 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/apremilast-treatment-in-early-oligoarticular-psoriatic-arthritis-improves-clinical-and-patient-reported-outcomes-for-up-to-48-weeks-data-from-a-phase-4-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apremilast-treatment-in-early-oligoarticular-psoriatic-arthritis-improves-clinical-and-patient-reported-outcomes-for-up-to-48-weeks-data-from-a-phase-4-trial/