Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
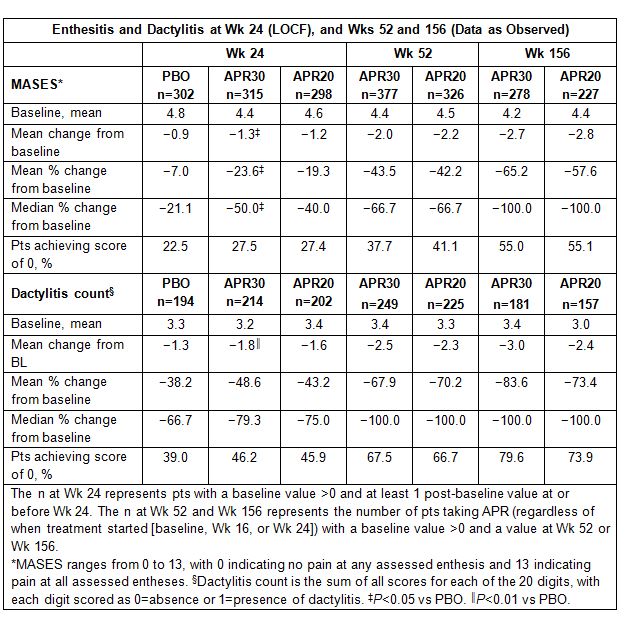
Of the multiple manifestations of psoriatic arthritis (PsA), enthesitis and dactylitis are hallmark features that affect many patients (pts), can lead to pain and disability, and may be particularly difficult to manage; the effectiveness of conventional DMARDs for these manifestations is not well documented. Apremilast (APR) is a phosphodiesterase 4 inhibitor that regulates immune responses in PsA; the phase III PALACE trials compared APR efficacy/safety with placebo (PBO) in pts with active PsA despite prior conventional DMARDs and/or biologics. We report the impact of APR treatment over 156 wks on enthesitis and dactylitis in a pooled analysis of the PALACE 1-3 studies.Methods: Pts were randomized (1:1:1) to PBO, APR 30 mg BID (APR30), or APR 20 mg BID (APR20) stratified by baseline DMARD use (yes/no). The PBO-controlled phase continued to Wk 24, with an early escape option at Wk 16. At Wk 24, all remaining PBO pts were re-randomized to APR30 or APR20. Double-blind APR treatment continued to Wk 52; pts could continue APR for up to an additional 4 years during an open-label extension phase. Data for pts entering the study with pre-existing enthesitis or dactylitis were pooled across PALACE 1-3, as pre-specified, to allow for a robust analysis. Enthesitis was evaluated based on the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) (range: 0-13), which indicates the number of painful entheses out of 13 enthesis sites. The dactylitis count (range: 0-20) is the number of digits (hands/feet) with dactylitis present (0=absence, 1=presence). Analyses at Wk 24 used LOCF for missing values and data for early escape pts; Wk 52 and Wk 156 were based on data as observed.
Results: Among pts with enthesitis (n=915) or dactylitis (n=610) at baseline and ≥1 post-baseline value, baseline mean MASES ranged from 4.4 to 4.8 and dactylitis counts ranged from 3.2 to 3.4. At Wk 24, mean change in MASES was −1.3 for APR30 (P=0.0194 vs PBO) and −0.9 for PBO, with 27.5% (APR30) and 22.5% (PBO) of pts achieving a MASES score of 0. Mean change in dactylitis count was −1.8 for APR30 (P=0.0097 vs PBO) and −1.3 for PBO, and a dactylitis count of 0 was achieved by 46.2% of APR30 and 39.0% of PBO pts. Sustained improvement in enthesitis and dactylitis severity was seen in pts who were receiving APR30 at Wk 156, as shown by reductions in MASES and dactylitis counts (Table). Mean % change in MASES with APR30 was −65.2%, and 55.0% of APR30 pts achieved a MASES score of 0. At Wk 156, mean % change in dactylitis count was −83.6% with APR30, and dactylitis counts decreased to 0 in 79.6% of pts. Data for APR20 demonstrated similar results.
Conclusion: The majority of pts (63%) entering the PALACE 1-3 studies had active enthesitis and 42% had dactylitis. APR30 BID demonstrated both early and long-term benefit, up to 156 wks, in treating enthesitis and dactylitis, including resolution of baseline disease in many pts.
To cite this abstract in AMA style:
Gladman DD, Kavanaugh A, Gomez-Reino JJ, Wollenhaupt J, Cutolo M, Schett G, Lespessailles E, McIlraith M, Hu C, Edwards CJ, Birbara CA, Mease PJ. Apremilast Treatment and Long-Term (156-Week) Improvements in Enthesitis and Dactylitis in Patients with Psoriatic Arthritis: Pooled Analysis of a Large Database of 3 Phase III, Randomized, Controlled Trials [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/apremilast-treatment-and-long-term-156-week-improvements-in-enthesitis-and-dactylitis-in-patients-with-psoriatic-arthritis-pooled-analysis-of-a-large-database-of-3-phase-iii-randomized-controlled/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apremilast-treatment-and-long-term-156-week-improvements-in-enthesitis-and-dactylitis-in-patients-with-psoriatic-arthritis-pooled-analysis-of-a-large-database-of-3-phase-iii-randomized-controlled/