Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Apremilast (APR), an oral phosphodiesterase 4 inhibitor, helps regulate the immune response that causes inflammation and skin disease associated with psoriatic arthritis (PsA). PALACE 4 compared the efficacy and safety of APR with placebo in patients with active PsA who were DMARD-naive.
Methods: Patients were randomized (1:1:1) to placebo, APR 20 mg BID (APR20), or APR 30 mg BID (APR30). Patients whose swollen and tender joint counts (SJC and TJC) had not improved by ≥20% at Week 16 were considered non-responders and were required to be re-randomized (1:1) to APR20 or APR30 if they were initially randomized to placebo, or continued on their initial APR dose. At Week 24, all remaining placebo patients were re-randomized to APR20 or APR30. The analysis comprises data from Weeks 0 to 52.
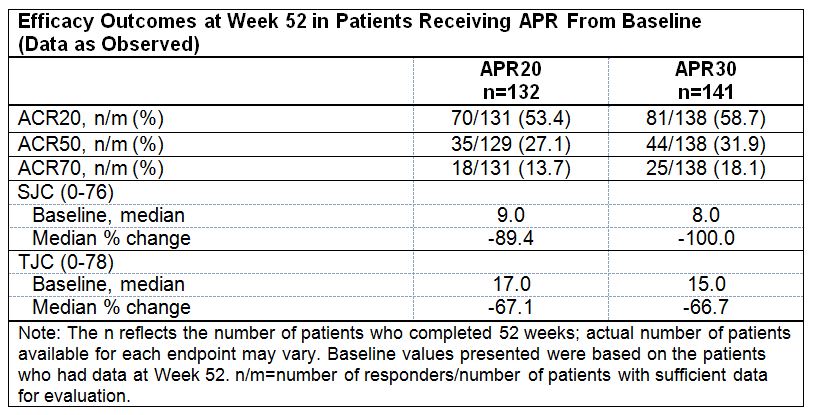
Results: At baseline, median PsA duration among patients in the full analysis set, overall, was 1.1 years; median SJC was 9.0 and median TJC was 16.0. At Week 16, significantly greater proportions of patients receiving APR achieved modified American College of Rheumatology 20 (ACR20) response (primary endpoint) (placebo: 15.9%; APR20: 28.0% [P=0.0062]; APR30: 30.7% [P=0.0010]) and modified ACR50 response (placebo: 4.5%; APR20: 11.4% [P=0.0173]; and APR30: 11.4% [P<0.0181]). ACR70 response was observed in 1.1%, 4.0%, and 4.0% of patients receiving placebo, APR20, and APR30, respectively. Median percent change in SJC at Week 16 was significantly greater with APR compared with placebo (placebo: -16.7%; APR20: -50.0% [P=0.0001]; APR30: -42.9% [P=0.0001]). Median percent change in TJC at Week 16 was also significantly improved with APR30 compared with placebo (placebo: -8.3%; APR20: -29.5% [P=0.0007]; APR30: -33.3% [P=0.0001]). At Week 52, modified ACR20/ACR50/ACR70 response was achieved by 53.4%/27.1%/13.7% and 58.7%/31.9%/18.1% of patients continually treated with APR20 or APR30, respectively, from baseline. Among these patients, improvements in SJC/TJC were sustained over 52 weeks; at Week 52 median percent change in SJC/TJC was -89.4%/-67.1% (APR20) and -100.0%/-66.7% (APR30). Consistent results were demonstrated in patients randomized to placebo at baseline and re-randomized to APR20 or APR30 at Week 16 or 24 who completed Week 52 (Table). The most common adverse events (AEs) reported among patients receiving either APR dose during the placebo-controlled period were nausea (12.6%), diarrhea (9.4%), and headache (6.0%). The nature and severity of AEs did not change with long-term exposure through 52 weeks.
Conclusion: Over 52 weeks, APR demonstrated clinically meaningful, sustained improvements in the signs and symptoms of PsA in DMARD-naïve patients. APR demonstrated an acceptable safety profile and was generally well tolerated.
Disclosure:
A. Wells,
Celgene Corporation,
2;
A. O. Adebajo,
None;
J. A. Aelion,
Ardea, Astra Zeneca, Bristol-Myers Squibb, Celgene Corporation, Centocor, Galapagos, Genentech, GlaxoSmithKline, Human Genome Sciences, Janssen, Eli Lilly, Merck, Mesoblast, Novartis, Novo Nordisk, Pfizer Inc, Roche, UCB Biosciences, Sanofi-Aventis, Taked,
2,
Ardea, Astra Zeneca, Bristol-Myers Squibb, Celgene Corporation, Centocor, Galapagos, Genentech, GlaxoSmithKline, Human Genome Sciences, Janssen, Eli Lilly, Merck, Mesoblast, Novartis, Novo Nordisk, Pfizer Inc, Roche, UCB Biosciences, Sanofi-Aventis, Taked,
5,
AbbVie, Amgen, and UCB,
8;
P. Bird,
Celgene Corporation,
2;
A. Kivitz,
Amgen, Janssen, Eli Lilly, Novartis, Pfizer Inc, and UCB,
2,
Amgen, Janssen, Eli Lilly, Novartis, Pfizer Inc, and UCB,
5,
Pfizer Inc,
8;
F. Lioté,
Celgene Corporation,
2,
Celgene Corporation,
5;
P. Sarzi-Puttini,
Abbvie, MSD, Roche, UCB and Alpha-Wassermann,
2;
C. Hu,
Celgene Corporation,
3,
Celgene Corporation,
1;
R. M. Stevens,
Celgene Corporation,
1,
Celgene Corporation,
3;
C. J. Edwards,
Celgene Corporation, Pfizer Inc, Roche, and Samsung,
2,
Celgene Corporation, Pfizer Inc, Roche, and Samsung,
5,
Abbott, Glaxo-SmithKline, Pfizer Inc, and Roche,
8.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apremilast-an-oral-phosphodiesterase-4-inhibitor-is-associated-with-long-term-52-week-improvement-in-the-signs-and-symptoms-of-psoriatic-arthritis-in-dmard-naive-patients-results-from-a-phase-3/