Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients (pts) with active
psoriatic arthritis (PsA) have disease involvement across multiple domains. Pts
can have substantial fatigue,
which is increasingly being recognized as important to pts, particularly those
with chronic diseases, and can affect QoL.1 The 2014 OMERACT PsA Working Group identified
fatigue measurement as an important outcome to consider including in PsA core
assessments.2 PALACE 1-3 compared apremilast (APR) efficacy/safety
with placebo (PBO) in pts with active PsA despite prior conventional DMARDs
and/or biologics, including assessment of fatigue levels. We report the impact
of APR treatment on fatigue over 104 wks in a pooled PALACE 1-3 analysis.
Methods: Pts were randomized (1:1:1) to PBO, APR
30 mg BID (APR30), or APR 20 mg BID (APR20) stratified by baseline DMARD use
(yes/no). The PBO-controlled phase continued to Wk 24, with a Wk 16 early
escape option. At Wk 24, all remaining PBO pts were re-randomized to APR30 or
APR20. Double-blind APR treatment continued to Wk 52; pts could then continue
to receive APR for up to an additional 4 years during an open-label extension
phase. Fatigue was assessed using the Functional Assessment of Chronic Illness
Therapy-Fatigue (FACIT-F) v4, a 13-item questionnaire initially developed to
assess anemia-associated fatigue.3 Questions are scored from 0-4. Total
FACIT-F scores range from 0-52, with higher scores denoting lower levels of fatigue.
As a point of reference, mean fatigue scores of 40.1-43.6 in the general
population, 35.8 in PsA patients,4 and 23.9 in anemic cancer pts have
been reported5,6; FACIT-F MCID in pts with rheumatoid
arthritis (RA) is 3-4.7
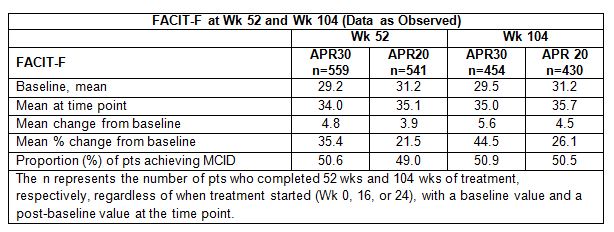
Results: Baseline mean FACIT-F scores in pts
receiving APR at Wks 52 and 104 ranged from 29.2-31.2, which are markedly below
population norms and indicative of fatigue-related impaired QOL. Long-term
improvement in fatigue was seen in APR pts at 52 wks, as shown by improvement
in FACIT-F score (Table). At Wk 104, APR30 pts had sustained improvements in
fatigue (mean FACIT-F=35.0), marking a shift toward population FACIT-F norms.
APR30 mean change was 5.6, which exceeded the MCID for this measure in RA pts; 50.9%
of APR30 pts achieved MCID for FACIT-F. APR30 mean % change in FACIT-F was
44.5%. Wk 104 findings were similar with APR20. Over 104 wks, most adverse
events (AEs) were mild/moderate; in general, no increase was seen in AE incidence/severity
with longer term exposure.
Conclusion:
Over 104 wks, APR
continued to improve fatigue in PsA pts. APR demonstrated an acceptable safety
profile and was generally well tolerated up to 104 wks.
References:
1. Swain. Clin Sci
(Lond). 2000;99:1-8. 2. Tillett et al. J Rheumatol. 2015 May 1.
[Epub]. 3. Yellen et al. J Pain Symptom Manage. 1997;13:63-74. 4.
Chandran et al. Ann Rheum Dis. 2007;66:936-9. 5. Webster et al. Health
Qual Life Outcomes. 2003;1:79. 6. Cella et al. Cancer.
2002;94:528-38. 7. Cella et al. J Rheumatol. 2005;32:811-9.
To cite this abstract in AMA style:
Kavanaugh A, Gladman D, Edwards CJ, Poder A, Liote F, Bird PA, Schett G, McIlraith M, Teng L, Mease PJ. Apremilast, an Oral Phosphodiesterase 4 Inhibitor, Is Associated with Long-Term (104-Week) Improvement in Fatigue in Patients with Psoriatic Arthritis: Pooled Results from 3 Phase 3, Randomized, Controlled Trials [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/apremilast-an-oral-phosphodiesterase-4-inhibitor-is-associated-with-long-term-104-week-improvement-in-fatigue-in-patients-with-psoriatic-arthritis-pooled-results-from-3-phase-3-randomized-contr/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apremilast-an-oral-phosphodiesterase-4-inhibitor-is-associated-with-long-term-104-week-improvement-in-fatigue-in-patients-with-psoriatic-arthritis-pooled-results-from-3-phase-3-randomized-contr/