Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Treat-to-target (T2T) management strategies in inflammatory rheumatic diseases aim to prevent damage and improve overall functioning and health by treating patients towards a predefined target reflecting inactive disease/low disease activity (ID/LDA). An international task force recommended to apply this strategy also in patients with SpA (1). T2T requires regular monitoring of disease activity. Currently, it is unknown what the uptake of these recommendations is in clinical practice. The objective of this study is to evaluate the extent to which T2T is applied in daily practice in patients with axial SpA (axSpA).
Methods: Data were used from three rheumatology centers participating in a quality registry for SpA in the Netherlands (SpA-Net) in which patient and physician relevant outcomes are collected electronically in daily practice (2). Patients were selected if diagnosed with axSpA before July 2017, enrolled in SpA-Net before 2018 and had ≥1 patient or physician reported outcome measure available throughout 2018 (January to December). The extent to which the T2T recommendations were followed was evaluated through four endpoints: First, in which proportion of patients disease activity was measured with ≥1 Ankylosing Spondylitis Disease Activity Score (ASDAS) annually. Second, in which proportion of patients with ≥1 measurement the target of ASDAS< 2.1 was achieved. Third, in which proportion of patients with high disease activity (=ASDAS≥2.1) the ASDAS was re-evaluated within 3, 6 or 12 months, and in patients with ID/LDA (=ASDAS< 2.1) within 6 or 12 months. Fourth, in which proportion of patients pharmacological treatment for SpA was adapted within 6 weeks after obtaining ASDAS≥2.1. The clinical characteristics of patients with high disease activity in whom treatment was adapted or not adapted were compared.
Results: In 185 out of 216 patients (86%) disease activity was measured with ≥1 annual ASDAS and in 71 out of these 185 (38%) patients the target was achieved at the first measurement. In patients with high disease activity, the score was re-evaluated within 3, 6 or 12 months in 26, 56 and 83 out of 114 patients (23%, 49% and 73%, respectively, figure 1). The proportion of patients in whom disease activity was re-evaluated within 12 months was the same in patients with ID/LDA and with high disease activity (73% and 73%, respectively). In 24 out of 114 (21%) patients with high disease activity at first measurement, treatment was adapted (figure 2). In this group, the ASDAS and physician global assessment were significantly higher compared to patients in whom treatment was not adapted (3.3 (SD 0.7) versus 3.0 (SD 0.6), p-value< 0.05 and 3.4 (SD 2.1) versus 1.6 (SD 1.1), p-value< 0.01, respectively, table 1).
Conclusion: Most patients with axSpA had ≥1 annual ASDAS recorded, but the target of ID/LDA was achieved in only one third of the patients at the first measurement. Subsequently, the scores do not seem to be used explicitly, as in patients with high disease activity re-evaluation of disease activity within recommended time periods and treatment adaptions occur only in a small proportion of patients. We conclude that T2T is applied only to a limited extent in clinical practice in patients with axSpA.
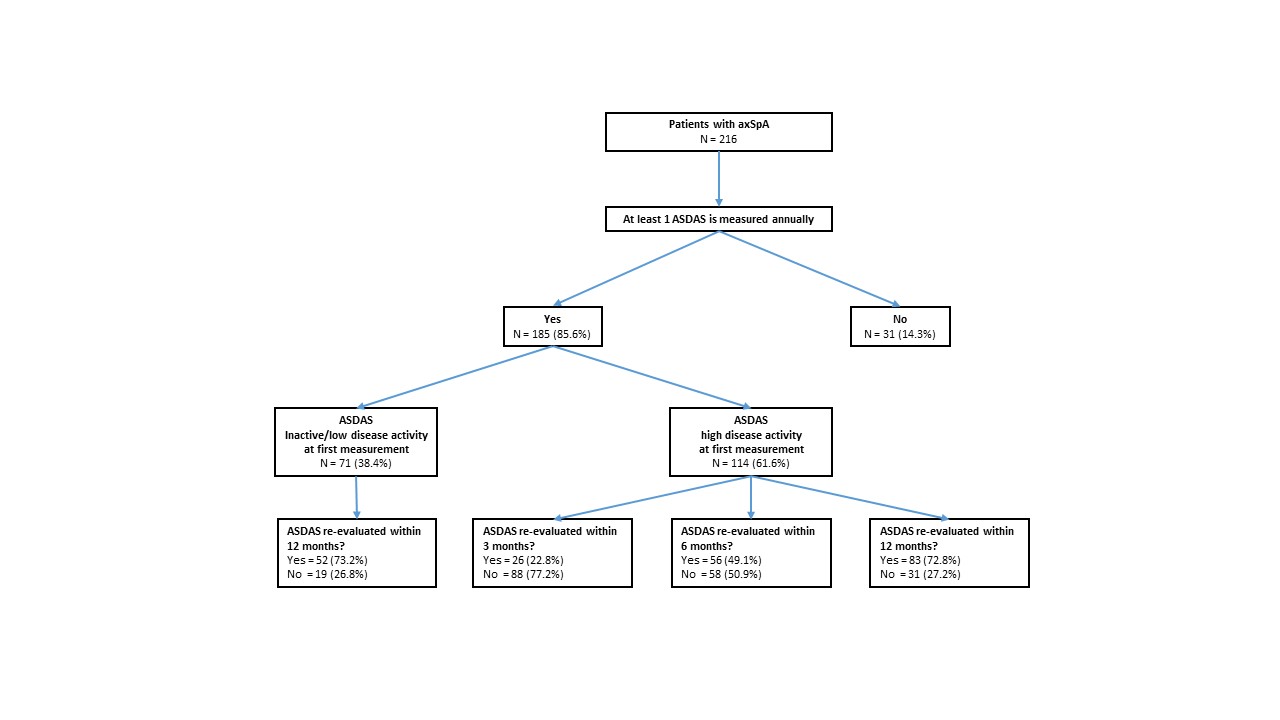 Figure 1 Flowchart of patients with axSpA and measurements of ASDAS within the study period in SpA-Net
Figure 1 Flowchart of patients with axSpA and measurements of ASDAS within the study period in SpA-Net
 Figure 2 Flowchart of patients, re-evaluation and treatment adaptations based on ASDAS scores
Figure 2 Flowchart of patients, re-evaluation and treatment adaptations based on ASDAS scores
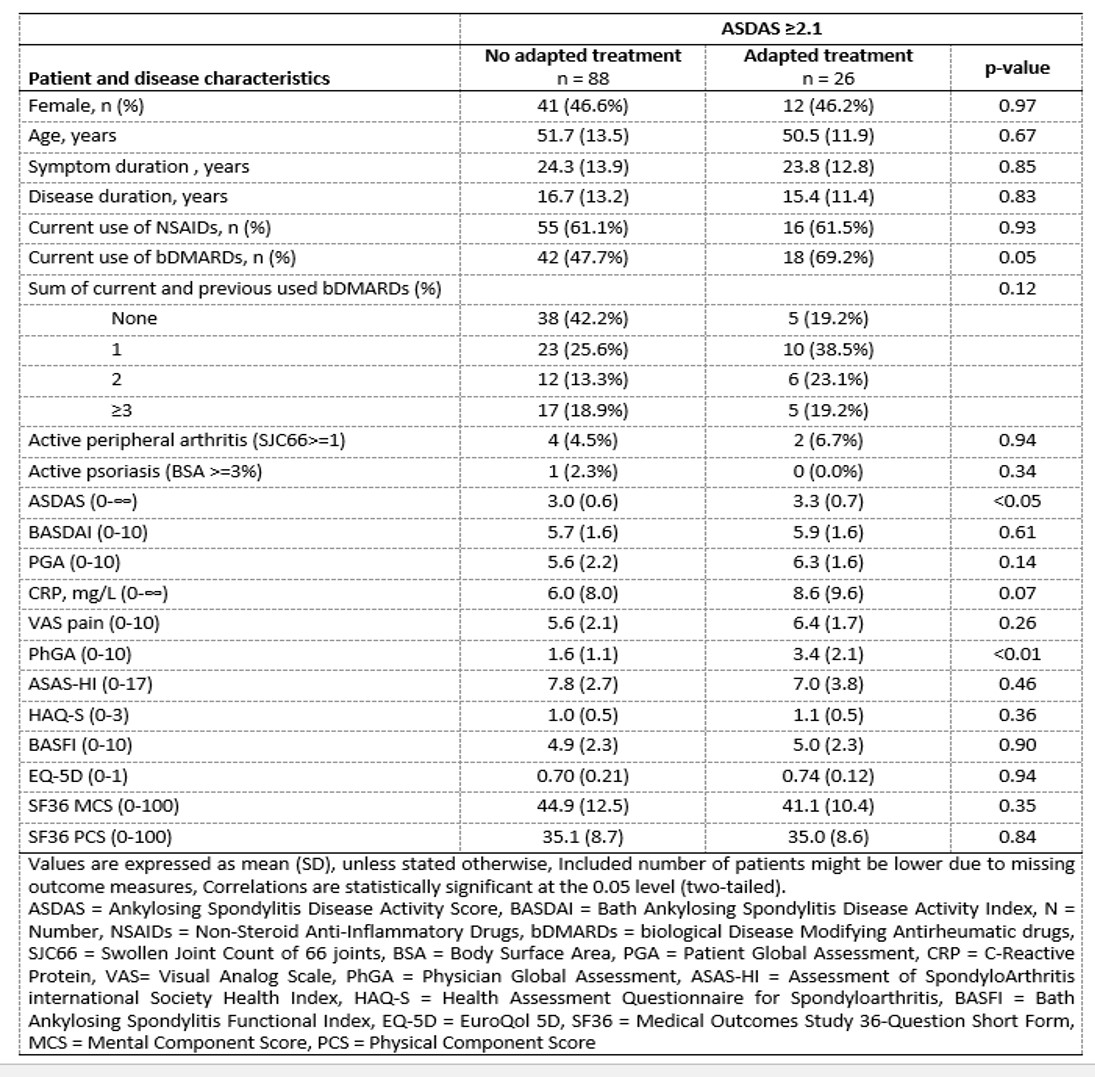 Table 1 Characteristics of patients with high disease activity in whom treatment was adapted or not
Table 1 Characteristics of patients with high disease activity in whom treatment was adapted or not
To cite this abstract in AMA style:
Beckers E, Boonen A, Webers C, Ten Klooster P, Vonkeman H, Efde M, van Tubergen A. Application of Treat-to-Target in Axial Spondyloarthritis in Daily Practice [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/application-of-treat-to-target-in-axial-spondyloarthritis-in-daily-practice/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/application-of-treat-to-target-in-axial-spondyloarthritis-in-daily-practice/
