Session Information
Date: Monday, October 27, 2025
Title: (1467–1516) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: With the introduction of anifrolumab to clinical practice, there is a growing interest in identifying factors associated with IFN activity in patients with SLE. An association between IFN gene signature and anti-U1-RNP antibody positivity has been reported. However, data from Asians are scarce. In this study, we investigated serological parameters associated with the IFN gene signature in patients with SLE, as well as its association with disease activity.
Methods: We utilized transcriptome data from 130 Japanese patients diagnosed with SLE from the ImmuNexUT database. IFN score was defined as the average normalized expression levels of IFI27, IFI44, IFI44L, and RSAD2. The associations between clinical parameters and the IFN score were assessed.
Results: The IFN scores showed a bimodal distribution; therefore, the patients were divided into two groups according to a cutoff score of 1.6. Of the 130 patients, 31 and 99 were classified into the low and high IFN groups, respectively. Clinical characteristics of patients in each group are shown in Table 1. Notably, disease activity was higher in the high IFN than the low IFN group (median [IQR] SLEDAI-2K score: 5 [2–9] vs. 2 [0–2], p < 0.0001). The IFN score was higher in patients positive for anti-U1-RNP antibodies (median [IQR]: 5.0 [4.3–5.4] vs. 2.8 [0.9–4.1]), anti-Sm antibodies (median [IQR]: 3.5 [1.4–4.7] vs. 5.1 [4.0–5.6]), anti-SSA antibodies (median [IQR]: 4.6 [3.7–5.3] vs. 1.4 [0.2–3.4]), anti-SSB antibodies (median [IQR]: 4.4 [3.5–5.6] vs. 3.7 [1.4–5.0]), and anti-dsDNA antibodies (median [IQR]: 4.5 [3.0–5.4] vs. 3.3 [1.2–4.7]) compared with patients negative for these autoantibodies (Figure 1A–E). Classification and regression tree analysis indicated anti-U1-RNP antibody positivity as the most important serological predictor of the IFN score (Figure 2A). Even among patients who are serologically active clinically quiescent (SACQ) and among patients who were both serologically and clinically inactive, the IFN score was higher in anti-U1-RNP antibody positive patients compared to negative patients (median [IQR]: 4.7 [4.1–5.1] vs. 1.4 [0.2–5.0], p = 0.0003 for SACQ, Figure 2B; median [IQR]: 4.5 [3.0–5.1] vs. 1.0 [0.3–2.6], p = 0.0002 for inactive, Figure 2C). Among SACQ patient and inactive patients, all patients positive for anti-U1-RNP antibodies belonged to the high IFN group while less than half of the patients negative for anti-U1-RNP antibodies belonged to the high IFN group (8/8 (100%) vs 10/21 (47.2%), p=0.03 for SACQ and 7/7 (100%) vs 8/22 (27.6%), p=0.01).
Conclusion: Autoantibody profiles, especially anti-U1-RNP antibodies, are useful for predicting IFN scores. Anti-U1-RNP antibody-positive patients have high IFN scores irrespective of disease activity.
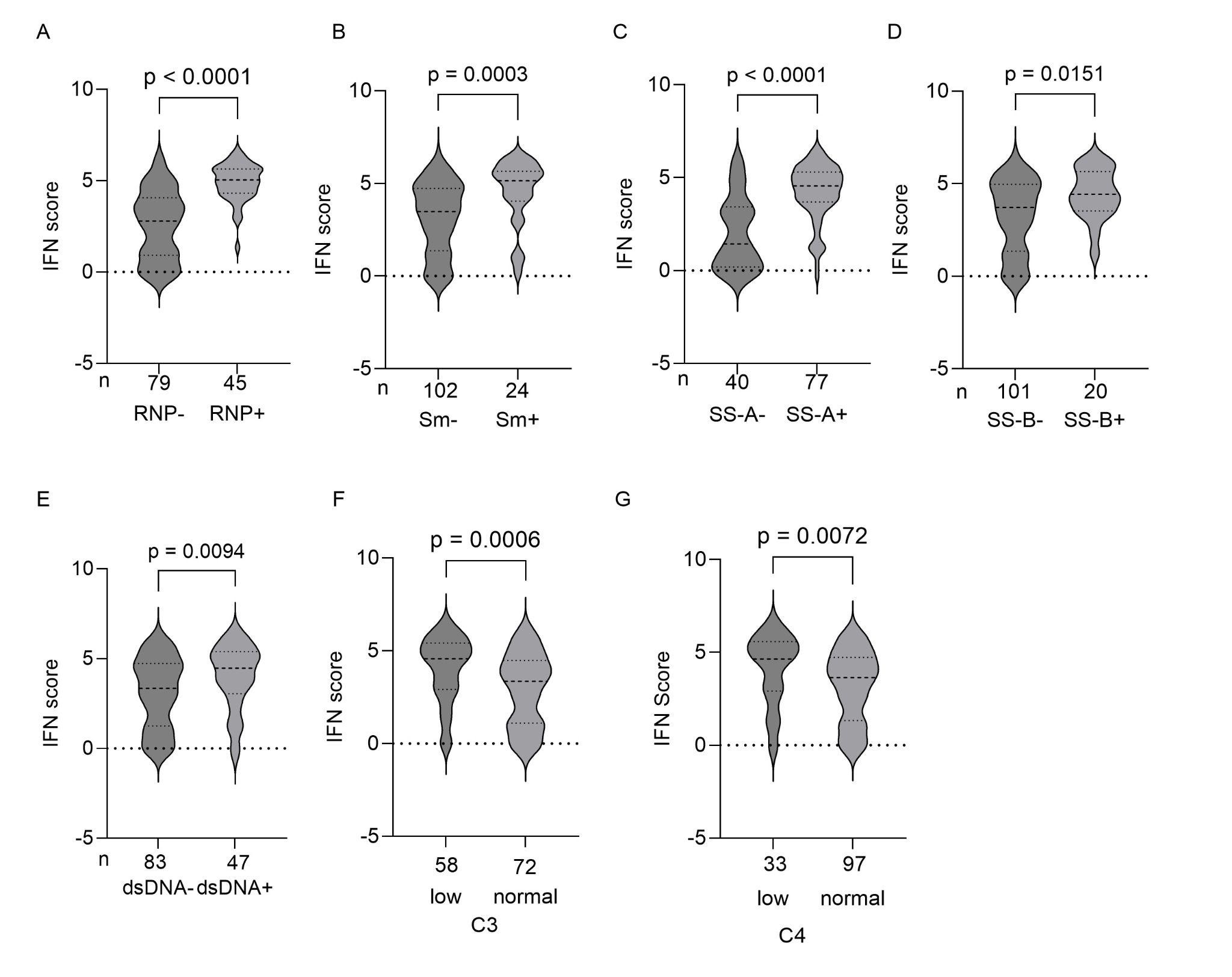 Figure 1: IFN Score and Serological Characteristics
Figure 1: IFN Score and Serological Characteristics
(A–E) IFN scores according to the anti-U1-RNP (A), anti-Sm (B), anti-SS-A (C), anti-SS-B (D), and anti-dsDNA (E) antibody statuses. (F and G) IFN scores according to C3 (F) and C4 (G) levels.
.jpg) Figure 2: The IFN score and anti-U1-RNP Antibody Status
Figure 2: The IFN score and anti-U1-RNP Antibody Status
(A) Classification and regression tree analysis of serological factors associated with the IFN score. The numbers in the boxes indicate the average IFN score in each group and the percentage of patients in that group. (B, C) IFN scores in serologically active, clinically quiescent patients (B) and inactive patients (C) according to anti-U1-RNP antibody status.
.jpg) Table 1: Clinical Characteristics of Patients in the Low and High IFN Groups
Table 1: Clinical Characteristics of Patients in the Low and High IFN Groups
To cite this abstract in AMA style:
Matsui T, Tsuchida Y, Itamiya T, Ota M, Komai T, Tsuchiya H, Shoda H, Okamura T, Fujio K. Anti-U1-RNP Antibody Positivity is Associated with Elevated Interferon Score in SLE Irrespective of Disease Activity: A Transcriptome Analysis in Japanese Patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/anti-u1-rnp-antibody-positivity-is-associated-with-elevated-interferon-score-in-sle-irrespective-of-disease-activity-a-transcriptome-analysis-in-japanese-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-u1-rnp-antibody-positivity-is-associated-with-elevated-interferon-score-in-sle-irrespective-of-disease-activity-a-transcriptome-analysis-in-japanese-patients/
