Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Up to half of pregnant patients with rheumatic diseases experience disease activity which can increase the risk of pregnancy complications and poor disease outcomes. Treatment with anti-tumor necrosis factor-alpha (anti-TNF-α) is extendedly used in these patients to control disease activity. Placental drug barrier cross has been described for all anti-TNF-α, except for certolizumab. It occurs mainly during the third trimester of pregnancy. Few studies have evaluated the changes of newborn’s immune system upon this exposure. The aim of this study was to evaluate the impact of anti-TNF-α exposure during pregnancy on the newborn’s immune system, especially on lymphocyte subpopulations and innate immunity (IL-12 / IFN-γ pathway).
Methods: Prospective multicenter study which included newborns born to mothers with rheumatic diseases (including rheumatoid arthritis, spondyloarthropaties (ankylosing, psoriatic), juvenile idiopathic arthritis) , treated during pregnancy with anti-TNF-α. A control group of newborns not exposed to anti-TNF-α during pregnancy was included. All neonates underwent: a) clinical follow-up by a pediatric immunologist in search for infections, allergies or autoimmune disease; b) Umbilical cord (UC) and peripheral blood analysis at birth, 3, 6 and 12 months to record lymphocyte T and B subpopulations, analyze lymphocytes’ proliferation capacity, as well as vaccine responses and innate immunity against mycobacteria. All patients signed informed consent.
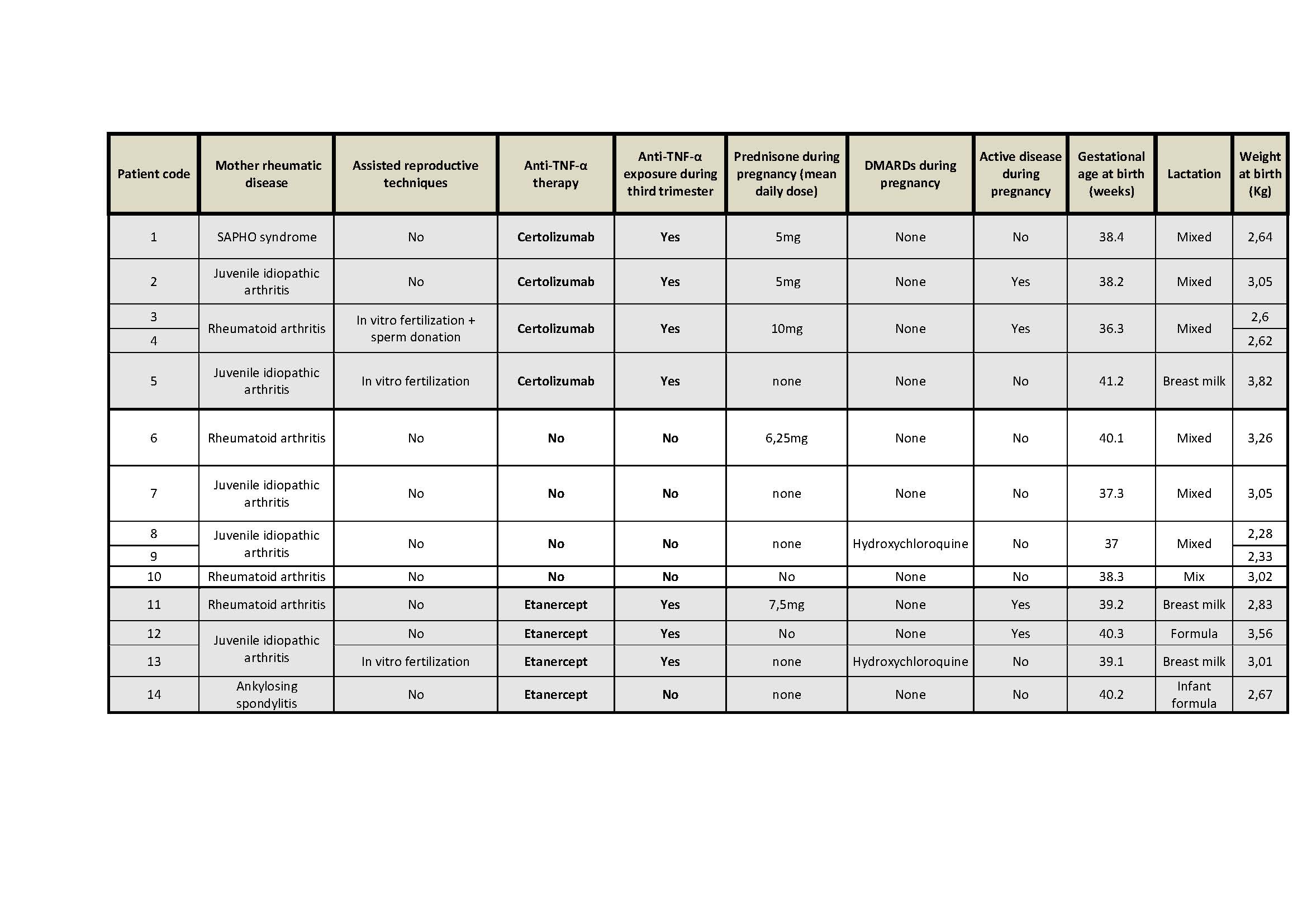
Results: Fourteen newborns have been included, 10 exposed to anti-TNF-α (Certolizumab: 6, throughout pregnancy; Etanercept: 4, of which 3 during throughout pregnancy) and 4 not exposed. Cohort features are summarized in table 1.
One child developed atopic dermatitis in the non-exposed group; no other allergies or autoimmune diseases have been reported. 7 infections (anti-TNF-α exposed: 3 vs non-exposed: 4) were diagnosed during follow-up. In 3 cases, the patient required hospitalization (1 pyelonephritis, 2 bronchiolitis).
Conclusion: The preliminary results do not show an increase in the risk of infections or immune dysregulation in the anti-TNF-α exposed group.
No significant quantitative differences are observed in the lymphogenesis, but a more immature B population and a predominantly T memory population was found in newborns exposed to anti-TNF-α. These differences are attenuated at 3 and 6 months. Functional capacity of the IL-12 / IFN-γ pathway seems intact. The ongoing recruitment will give consistency to these results.
To cite this abstract in AMA style:
Luo Y, Pluma A, Castellanos-Moreira R, Moreno E, Baños N, Rodriguez-Garcia S, Deyà-Martínez A, Garcia-Garcia A, Torres M, Grados D, Casellas M, Juan M, Esteve-Sole A, Alsina L. Anti-TNF-α Exposure During Pregnancy: Impact on the Neonate’s Immune System [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/anti-tnf-%ce%b1-exposure-during-pregnancy-impact-on-the-neonates-immune-system/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-tnf-%ce%b1-exposure-during-pregnancy-impact-on-the-neonates-immune-system/